Korean J Gastroenterol.
2015 Jul;66(1):5-9. 10.4166/kjg.2015.66.1.5.
New Therapeutic Agent for Chronic Hepatitis C: Direct Acting Agent
- Affiliations
-
- 1Department of Internal Medicine, Chungbuk National University College of Medicine and Medical Research Institute, Cheongju, Korea. hbchae@chungbuk.ac.kr
- KMID: 2373305
- DOI: http://doi.org/10.4166/kjg.2015.66.1.5
Abstract
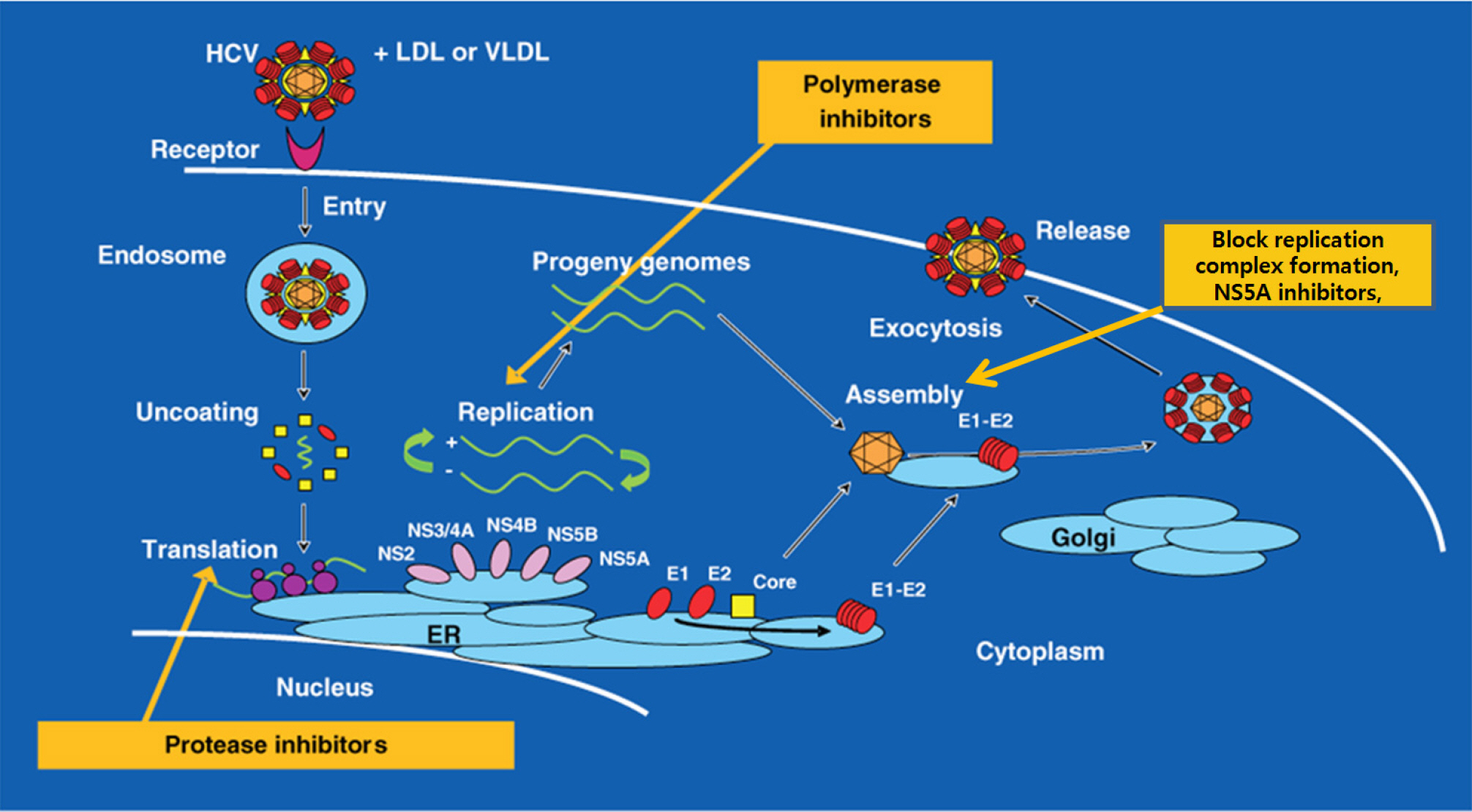
- Peg-interferon and ribavirin has been the standard therapy of chronic hepatitis C for the past 15 years in Korea. However, the treatment paradigm is changing. Direct acting agents (DAAs) are oral pills that can be easily taken. In addition, DAAs are more effective and have less adverse reactions compared to the previously used drugs. Chronic hepatitis C is hard to treat because the virus is error-prone virus. Host immunity is helpless against the hepatitis C virus since it evades the host immunity through various complex mechanisms. There are 6 genotypes. Quasispecies can co-exist even in the same patients. The treatment strategy is based on the combination of the individual drug corresponding to each step of viral replication process. NS5B nucleosides are the most powerful and effective drug available until now. Other drugs with different mechanisms of action can be used to provide synergy. NS5A and NS5B inhibition drugs currently belong to the leading group amongst many DAAs. These drugs will soon be available in Korea. We have to know the merits and adverse drug reactions of the new drug.
Keyword
MeSH Terms
-
Antiviral Agents/*therapeutic use
Drug Therapy, Combination
Enzyme Inhibitors/therapeutic use
Genotype
Guidelines as Topic
Hepacivirus/genetics
Hepatitis C, Chronic/*drug therapy/immunology/virology
Humans
Viral Nonstructural Proteins/antagonists & inhibitors/metabolism
Antiviral Agents
Enzyme Inhibitors
Viral Nonstructural Proteins
Figure
Reference
-
References
1. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon al-fa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001; 358:958–965.
Article2. Hadziyannis SJ, Sette H Jr, Morgan TR, et al. PEGASYS International Study Group. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004; 140:346–355.3. Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut. 2006; 55:1350–1359.
Article4. Chang KM, Rehermann B, McHutchison JG, et al. Immunological significance of cytotoxic T lymphocyte epitope variants in patients chronically infected by the hepatitis C virus. J Clin Invest. 1997; 100:2376–2385.
Article5. Weiner A, Erickson AL, Kansopon J, et al. Persistent hepatitis C virus infection in a chimpanzee is associated with emergence of a cytotoxic T lymphocyte escape variant. Proc Natl Acad Sci U S A. 1995; 92:2755–2759.
Article6. Tsai SL, Chen YM, Chen MH, et al. Hepatitis C virus variants cir-cumventing cytotoxic T lymphocyte activity as a mechanism of chronicity. Gastroenterology. 1998; 115:954–965.
Article7. Shin EC, Rehermann B. Taking the brake off T cells in chronic viral infection. Nat Med. 2006; 12:276–277.
Article8. Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004; 22:531–562.
Article9. Jacobson IM, McHutchison JG, Dusheiko G, et al. ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med. 2011; 364:2405–2416.
Article10. Poordad F, McCone J Jr, Bacon BR, et al. SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med. 2011; 364:1195–1206.
Article11. Hézode C, Fontaine H, Dorival C, et al. CUPIC Study Group. Triple therapy in treatmentexperienced patients with HCV-cirrhosis in a multicentre cohort of the French Early Access Programme (ANRS CO20-CUPIC) – NCT01514890. J Hepatol. 2013; 59:434–441.
Article12. Chae HB, Park SM, Youn SJ. Direct-acting antivirals for the treatment of chronic hepatitis C: open issues and future perspectives. ScientificWorldJournal. 2013. DOI: doi:10.1155/2013/704912.
Article13. Racanelli V, Rehermann B. Hepatitis C virus infection: when si-lence is deception. Trends Immunol. 2003; 24:456–464.
Article14. Koff RS. Review article: the efficacy and safety of sofosbuvir, a novel, oral nucleotide NS5B polymerase inhibitor, in the treatment of chronic hepatitis C virus infection. Aliment Pharmacol Ther. 2014; 39:478–487.
Article15. Afdhal N, Zeuzem S, Kwo P, et al. ION-1 Investigators. Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med. 2014; 370:1889–1898.
Article16. Afdhal N, Reddy KR, Nelson DR, et al. ION-2 Investigators. Ledipasvir and sofosbuvir for previously treated HCV genotype 1 infection. N Engl J Med. 2014; 370:1483–1493.
Article17. Kowdley KV, Gordon SC, Reddy KR, et al. ION-3 Investigators. Ledipasvir and sofosbuvir for 8 or 12 weeks for chronic HCV without cirrhosis. N Engl J Med. 2014; 370:1879–1888.
Article18. Jacobson IM, Ghalib RH, Rodriguez-Torres M, et al. SVR results of a once-daily regimen of simprevir (TMC435) plus sofosbuvir (GS-7977) with or without ribavirin in cirrhotic and noncirrhotic HCV genotype 1 treatmentnaive and prior null responder patients: The COSMOS study. Hepatology. 2013; 58:1379A.19. Kowdley KV, Lawitz E, Poordad F, et al. Safety and efficacy of interferon-free regiments of ABT-450/r, ABT-267, ABT-333 +/- ribavirin in patients with chronic HCV GT1 infection: results from the aviator study. J Hepatol. 2013; 58:S2.20. Manns M, Pol S, Jacobson IM, et al. HALLMARK-DUAL Study Team. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 2014; 384:1597–1605.
Article21. American Association for the Study of Liver Diseases and the Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C [Internet]. Alexandria (VA): American Association for the Study of Liver Diseases and the Infectious Diseases Society of America;c2014. [cited 2014 Mar 12]. Available from:. http://www.hcvguidelines.org/full-report-view.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- 2017 KASL clinical practice guidelines management of hepatitis C: Treatment of chronic hepatitis C
- Treatment of Chronic Hepatitis C Patients Who Do Not Respond to Direct Acting Antivirals
- Management of hepatitis C viral infection in chronic kidney disease patients on hemodialysis in the era of direct-acting antivirals
- New antiviral agents for treatment of chronic hepatitis B
- 2017 Korean Association for the Study of the Liver (KASL) Clinical Practice Guidelines of Chronic Hepatitis C: What's New?