Korean J Physiol Pharmacol.
2017 Jan;21(1):91-97. 10.4196/kjpp.2017.21.1.91.
Effects of high glucose with or without other metabolic substrates on alpha-adrenergic contractions in rat mesenteric and femoral arteries
- Affiliations
-
- 1Chung-Ang University Red Cross College of Nursing, Seoul 06974, Korea. hyoo@cau.ac.kr
- 2Chung-Ang University Graduate School, Seoul 06974, Korea.
- KMID: 2371072
- DOI: http://doi.org/10.4196/kjpp.2017.21.1.91
Abstract
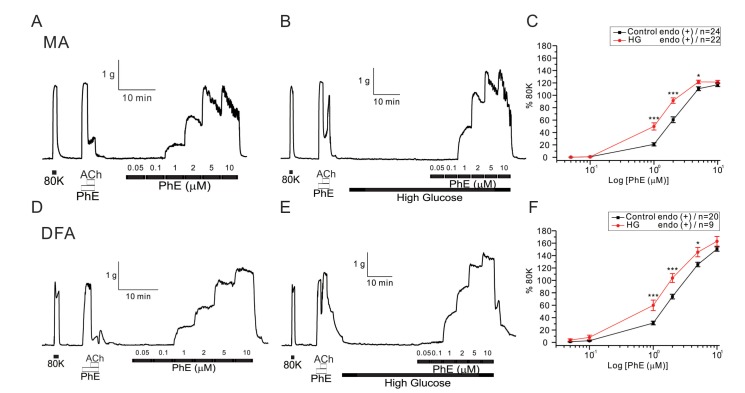
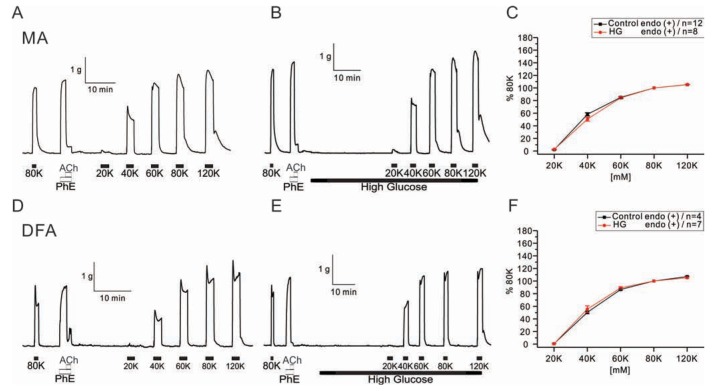
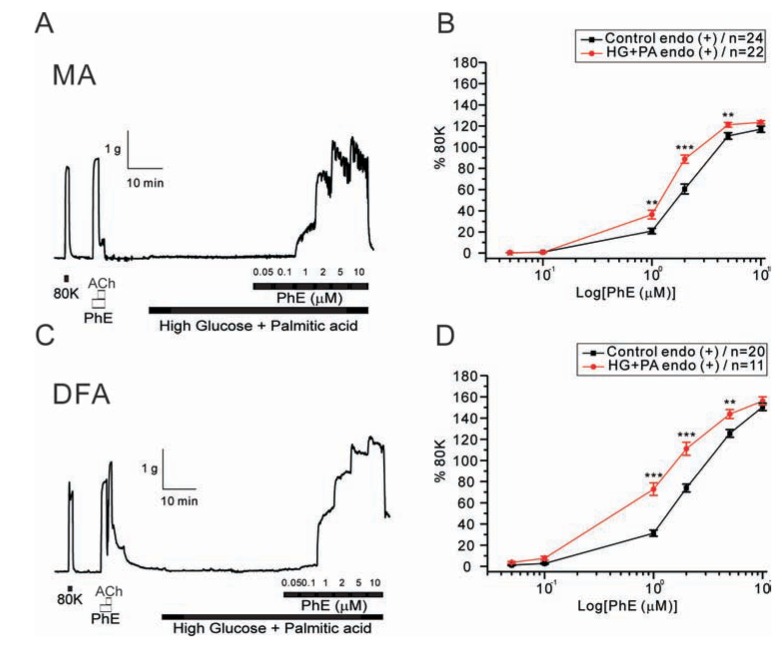
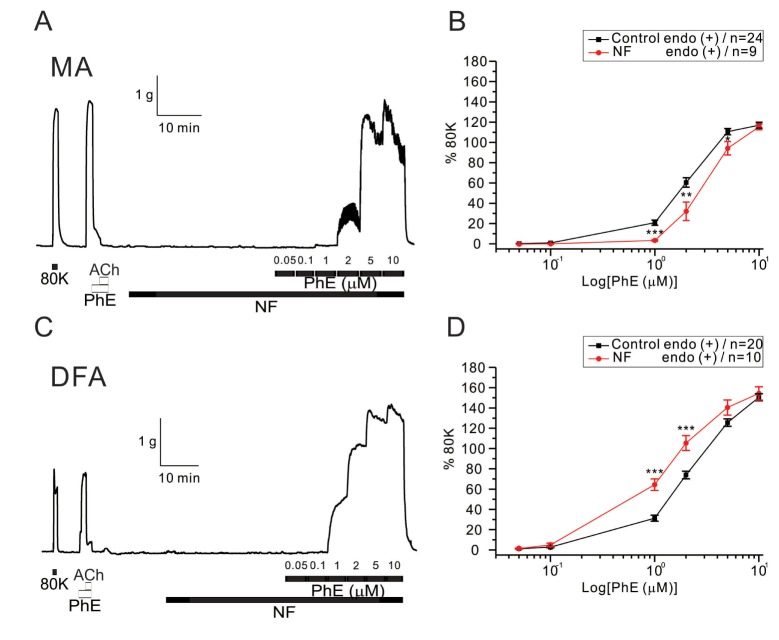
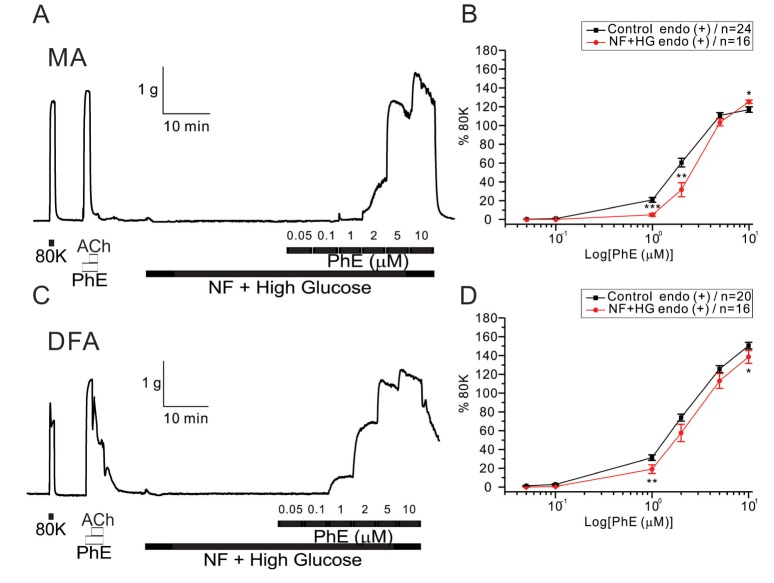
- Hyperglycemia is associated with an increased risk of cardiovascular diseases. It has been demonstrated that chronic exposure to high glucose impaired endothelial functions. However, specific effects of short-term exposure to high glucose on vascular reactivity are controversial. Moreover, the combined effects of other metabolic substrates such as free fatty acids (FFA) on vascular reactivity remain poorly understood. Here we investigate the effects of short-term exposure to high glucose with or without other metabolic substrates including FFAs termed "nutrition full" (NF) solution, on mesenteric (MA) and deep femoral arteries (DFA) of rats. Arterial ring segments were mounted in a double-wire myograph. Contraction in response to phenylephrine (PhE) was determined in control (5 mM) and high glucose (23 mM, HG) environments over a 30 min period. In both arteries, PhE-inducedvasocontraction was enhanced by pre-incubation of HG solution. A combined incubation with HG and palmitic acid (100 µM) induced similar sensitization of PhE-contractions in both arteries. In contrast, high Kâº-induced contractions were not affected by HG. Interestingly, pre-incubation with NF solution decreased PhE-induced contraction in MA but increased the contraction in DFA. In NF solution, the HG-induced facilitation of PhE-contraction was not observed in MA. Furthermore, the PhE-induced contraction of DFA was attenuated by HG in NF solution. Our results demonstrate that the sensitization of PhE-induced arterial contraction by HG is differentially affected by other metabolic substrates. The conversation of skeletal arterial contractility by HG in NF solution requires careful interpretation of the previous in vitro studies where only glucose is included in physiological salt solutions. Further studies are required to elucidate the mechanism underlying the inconsistent effect of NF solution on MA and DFA.
MeSH Terms
Figure
Cited by 1 articles
-
Differential effects of saturated and unsaturated fatty acids on vascular reactivity in isolated mesenteric and femoral arteries of rats
Rany Vorn, Hae Young Yoo
Korean J Physiol Pharmacol. 2019;23(5):403-409. doi: 10.4196/kjpp.2019.23.5.403.
Reference
-
1. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clinical Diabetes. 2008; 26:77–82.
Article2. Triggle CR, Samuel SM, Ravishankar S, Marei I, Arunachalam G, Ding H. The endothelium: influencing vascular smooth muscle in many ways. Can J Physiol Pharmacol. 2012; 90:713–738. PMID: 22625870.
Article3. Sandoo A, van Zanten JJ, Metsios GS, Carroll D, Kitas GD. The endothelium and its role in regulating vascular tone. Open Cardiovasc Med J. 2010; 4:302–312. PMID: 21339899.
Article4. Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, Nishigaki I. The vascular endothelium and human diseases. Int J Biol Sci. 2013; 9:1057–1069. PMID: 24250251.
Article5. Taylor PD, Poston L. The effect of hyperglycaemia on function of rat isolated mesenteric resistance artery. Br J Pharmacol. 1994; 113:801–808. PMID: 7858870.
Article6. Akbari CM, Saouaf R, Barnhill DF, Newman PA, LoGerfo FW, Veves A. Endothelium-dependent vasodilatation is impaired in both microcirculation and macrocirculation during acute hyperglycemia. J Vasc Surg. 1998; 28:687–694. PMID: 9786265.
Article7. Brouwers O, Niessen PM, Haenen G, Miyata T, Brownlee M, Stehouwer CD, De Mey JG, Schalkwijk CG. Hyperglycaemia-induced impairment of endothelium-dependent vasorelaxation in rat mesenteric arteries is mediated by intracellular methylglyoxal levels in a pathway dependent on oxidative stress. Diabetologia. 2010; 53:989–1000. PMID: 20186387.
Article8. Zhou ZW, Xie XL, Zhou SF, Li CG. Mechanism of reversal of high glucose-induced endothelial nitric oxide synthase uncoupling by tanshinone IIA in human endothelial cell line EA.hy926. Eur J Pharmacol. 2012; 697:97–105. PMID: 23063542.
Article9. Koo JR, Ni Z, Oviesi F, Vaziri ND. Antioxidant therapy potentiates antihypertensive action of insulin in diabetic rats. Clin Exp Hypertens. 2002; 24:333–344. PMID: 12109774.
Article10. Marfella R, Quagliaro L, Nappo F, Ceriello A, Giugliano D. Acute hyperglycemia induces an oxidative stress in healthy subjects. J Clin Invest. 2001; 108:635–636. PMID: 11518739.
Article11. Li PA, Liu GJ, He QP, Floyd RA, Siesjö BK. Production of hydroxyl free radical by brain tissues in hyperglycemic rats subjected to transient forebrain ischemia. Free Radic Biol Med. 1999; 27:1033–1040. PMID: 10569636.
Article12. Hsieh TJ, Zhang SL, Filep JG, Tang SS, Ingelfinger JR, Chan JS. High glucose stimulates angiotensinogen gene expression via reactive oxygen species generation in rat kidney proximal tubular cells. Endocrinology. 2002; 143:2975–2985. PMID: 12130563.
Article13. Li H, Chai Q, Gutterman DD, Liu Y. Elevated glucose impairs cAMP-mediated dilation by reducing Kv channel activity in rat small coronary smooth muscle cells. Am J Physiol Heart Circ Physiol. 2003; 285:H1213–H1219. PMID: 12763748.14. El-Awady MS, El-Agamy DS, Suddek GM, Nader MA. Propolis protects against high glucose-induced vascular endothelial dysfunction in isolated rat aorta. J Physiol Biochem. 2014; 70:247–254. PMID: 24234058.
Article15. MacKenzie A, Cooper EJ, Dowell FJ. Differential effects of glucose on agonist-induced relaxations in human mesenteric and subcutaneous arteries. Br J Pharmacol. 2008; 153:480–487. PMID: 18037911.
Article16. Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, Creager MA. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998; 97:1695–1701. PMID: 9591763.
Article17. Chinen I, Shimabukuro M, Yamakawa K, Higa N, Matsuzaki T, Noguchi K, Ueda S, Sakanashi M, Takasu N. Vascular lipotoxicity: endothelial dysfunction via fatty-acid-induced reactive oxygen species overproduction in obese Zucker diabetic fatty rats. Endocrinology. 2007; 148:160–165. PMID: 17023526.
Article18. Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000; 404:787–790. PMID: 10783895.
Article19. Saraswathi V, Wu G, Toborek M, Hennig B. Linoleic acid-induced endothelial activation: role of calcium and peroxynitrite signaling. J Lipid Res. 2004; 45:794–804. PMID: 14993245.20. Jaimes EA, Hua P, Tian RX, Raij L. Human glomerular endothelium: interplay among glucose, free fatty acids, angiotensin II, and oxidative stress. Am J Physiol Renal Physiol. 2010; 298:F125–F132. PMID: 19864304.
Article21. Sainsbury CA, Sattar N, Connell JM, Hillier C, Petrie JR. Non-esterified fatty acids impair endothelium-dependent vasodilation in rat mesenteric resistance vessels. Clin Sci (Lond). 2004; 107:625–629. PMID: 15367101.
Article22. Bhagavan NV, Ha JS, Park JH, Honda SA, Rios CN, Sugiyama C, Fujitani GK, Takeuchi IK, Ha CE. Utility of serum Fatty Acid concentrations as a marker for acute myocardial infarction and their potential role in the formation of ischemia-modified albumin: a pilot study. Clin Chem. 2009; 55:1588–1590. PMID: 19498051.
Article23. Banskota AH, Tezuka Y, Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001; 15:561–571. PMID: 11746834.
Article24. Qian L, Wang H, Xia Q, Bruce I, Huang H. Interleukin-2 improves vascular functions in streptozotocin-induced diabetic rats. In : 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference; 2005 17-18 Jan; 2006.25. Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013; 34:2436–2443. PMID: 23641007.
Article26. Gerich JE. Clinical significance, pathogenesis, and management of postprandial hyperglycemia. Arch Intern Med. 2003; 163:1306–1316. PMID: 12796066.
Article27. Endemann DH, Schiffrin EL. Endothelial dysfunction. J Am Soc Nephrol. 2004; 15:1983–1992. PMID: 15284284.
Article28. Beckman JA, Goldfine AB, Gordon MB, Creager MA. Ascorbate restores endothelium-dependent vasodilation impaired by acute hyperglycemia in humans. Circulation. 2001; 103:1618–1623. PMID: 11273987.
Article29. Ahmad M, Turkseven S, Mingone CJ, Gupte SA, Wolin MS, Abraham NG. Heme oxygenase-1 gene expression increases vascular relaxation and decreases inducible nitric oxide synthase in diabetic rats. Cell Mol Biol (Noisy-le-grand). 2005; 51:371–376. PMID: 16309587.30. Ozkan MH, Uma S. Inhibition of acetylcholine-induced EDHF response by elevated glucose in rat mesenteric artery. Life Sci. 2005; 78:14–21. PMID: 16125203.31. Rajagopalan S, Kurz S, Münzel T, Tarpey M, Freeman BA, Griendling KK, Harrison DG. Angiotensin II-mediated hypertension in the rat increases vascular superoxide production via membrane NADH/NADPH oxidase activation. Contribution to alterations of vasomotor tone. J Clin Invest. 1996; 97:1916–1923. PMID: 8621776.
Article32. Inoguchi T, Li P, Umeda F, Yu HY, Kakimoto M, Imamura M, Aoki T, Etoh T, Hashimoto T, Naruse M, Sano H, Utsumi H, Nawata H. High glucose level and free fatty acid stimulate reactive oxygen species production through protein kinase C--dependent activation of NAD(P)H oxidase in cultured vascular cells. Diabetes. 2000; 49:1939–1945. PMID: 11078463.
Article33. Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Protein kinase C-dependent increase in reactive oxygen species (ROS) production in vascular tissues of diabetes: role of vascular NAD(P)H oxidase. J Am Soc Nephrol. 2003; 14(8 Suppl 3):S227–S232. PMID: 12874436.
Article34. Tomasian D, Keaney JF, Vita JA. Antioxidants and the bioactivity of endothelium-derived nitric oxide. Cardiovasc Res. 2000; 47:426–435. PMID: 10963716.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential effects of saturated and unsaturated fatty acids on vascular reactivity in isolated mesenteric and femoral arteries of rats
- The Role of beta-Adrenergic Receptor in the Seminal Vesicle Contraction
- Clinically relevant concentrations of dexmedetomidine may reduce oxytocin-induced myometrium contractions in pregnant rats
- The diabetes-induced functional and distributional changes of the alpha 1-adrenoceptor of the abdominal aorta and distal mesenteric artery from streptozotocin-induced diabetic rats
- Gender difference and change of alpha1-adrenoceptors in the distal mesenteric arteries of streptozotocin-induced diabetic rats