Korean J Physiol Pharmacol.
2017 Mar;21(2):169-177. 10.4196/kjpp.2017.21.2.169.
Lamotrigine, an antiepileptic drug, inhibits 5-HT₃ receptor currents in NCB-20 neuroblastoma cells
- Affiliations
-
- 1Department of Physiology, Augusta University, Augusta, GA 30912, USA.
- 2Department of Pharmacology, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. sungkw@catholic.ac.kr
- KMID: 2371035
- DOI: http://doi.org/10.4196/kjpp.2017.21.2.169
Abstract
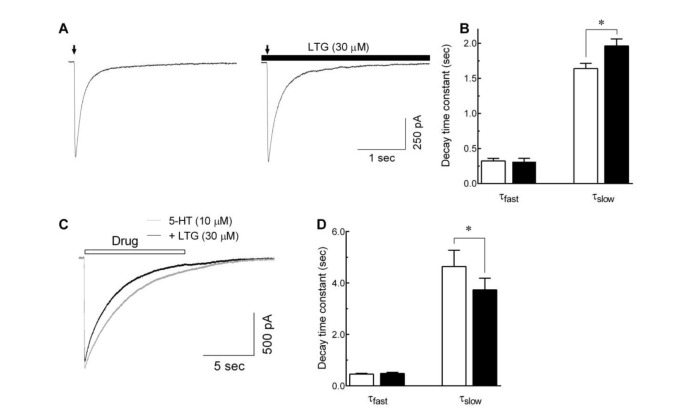
- Lamotrigine is an antiepileptic drug widely used to treat epileptic seizures. Using whole-cell voltage clamp recordings in combination with a fast drug application approach, we investigated the effects of lamotrigine on 5-hydroxytryptamine (5-HT)₃ receptors in NCB-20 neuroblastoma cells. Co-application of lamotrigine (1~300 µM) resulted in a concentration-dependent reduction in peak amplitude of currents induced by 3 µM of 5-HT for an ICâ‚…â‚€ value of 28.2±3.6 µM with a Hill coefficient of 1.2±0.1. These peak amplitude decreases were accompanied by the rise slope reduction. In addition, 5-HT₃-mediated currents evoked by 1 mM dopamine, a partial 5-HT₃ receptor agonist, were inhibited by lamotrigine co-application. The ECâ‚…â‚€ of 5-HT for 5-HT₃ receptor currents were shifted to the right by co-application of lamotrigine without a significant change of maximal effect. Currents activated by 5-HT and lamotrigine co-application in the presence of 1 min pretreatment of lamotrigine were similar to those activated by 5-HT and lamotrigine co-application alone. Moreover, subsequent application of lamotrigine in the presence of 5-HT and 5-hydroxyindole, known to attenuate 5-HT₃ receptor desensitization, inhibited 5-HT₃ receptor currents in a concentration-dependent manner. The deactivation of 5-HT₃ receptor was delayed by washing with an external solution containing lamotrigine. Lamotrigine accelerated the desensitization process of 5-HT₃ receptors. There was no voltage-dependency in the inhibitory effects of lamotrigine on the 5-HT3 receptor currents. These results indicate that lamotrigine inhibits 5-HT₃-activated currents in a competitive manner by binding to the open state of the channels and blocking channel activation or accelerating receptor desensitization.
MeSH Terms
Figure
Reference
-
1. Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999; 38:1083–1152. PMID: 10462127.
Article2. Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002; 71:533–554. PMID: 11888546.
Article3. Thompson AJ, Lummis SC. The 5-HT3 receptor as a therapeutic target. Expert Opin Ther Targets. 2007; 11:527–540. PMID: 17373882.4. Walstab J, Rappold G, Niesler B. 5-HT(3) receptors: role in disease and target of drugs. Pharmacol Ther. 2010; 128:146–169. PMID: 20621123.
Article5. Barnes NM, Hales TG, Lummis SC, Peters JA. The 5-HT3 receptor--the relationship between structure and function. Neuropharmacology. 2009; 56:273–284. PMID: 18761359.6. Thompson AJ. Recent developments in 5-HT3 receptor pharmacology. Trends Pharmacol Sci. 2013; 34:100–109. PMID: 23380247.7. Walstab J, Rappold G, Niesler B. 5-HT(3) receptors: role in disease and target of drugs. Pharmacol Ther. 2010; 128:146–169. PMID: 20621123.
Article8. Jones BJ, Blackburn TP. The medical benefit of 5-HT research. Pharmacol Biochem Behav. 2002; 71:555–568. PMID: 11888547.
Article9. Harmer CJ, Reid CB, Ray MK, Goodwin GM, Cowen PJ. 5HT(3) antagonism abolishes the emotion potentiated startle effect in humans. Psychopharmacology (Berl). 2006; 186:18–24. PMID: 16572265.
Article10. Brunello N, Masotto C, Steardo L, Markstein R, Racagni G. New insights into the biology of schizophrenia through the mechanism of action of clozapine. Neuropsychopharmacology. 1995; 13:177–213. PMID: 8602893.
Article11. Wada Y, Shiraishi J, Nakamura M, Koshino Y. Effects of the 5-HT3 receptor agonist 1-(m-chlorophenyl)-biguanide in the rat kindling model of epilepsy. Brain Res. 1997; 759:313–316. PMID: 9221955.12. Nichols RA, Mollard P. Direct observation of serotonin 5-HT3 receptor-induced increases in calcium levels in individual brain nerve terminals. J Neurochem. 1996; 67:581–592. PMID: 8764583.13. Michel K, Zeller F, Langer R, Nekarda H, Kruger D, Dover TJ, Brady CA, Barnes NM, Schemann M. Serotonin excites neurons in the human submucous plexus via 5-HT3 receptors. Gastroenterology. 2005; 128:1317–1326. PMID: 15887114.14. Bialer M, Johannessen SI, Kupferberg HJ, Levy RH, Perucca E, Tomson T. Progress report on new antiepileptic drugs: a summary of the Eigth Eilat Conference (EILAT VIII). Epilepsy Res. 2007; 73:1–52. PMID: 17158031.
Article15. Rho JM, Sankar R. The pharmacologic basis of antiepileptic drug action. Epilepsia. 1999; 40:1471–1483. PMID: 10565572.
Article16. Vallés AS, Garbus I, Barrantes FJ. Lamotrigine is an open-channel blocker of the nicotinic acetylcholine receptor. Neuroreport. 2007; 18:45–50. PMID: 17259859.
Article17. Zheng C, Yang K, Liu Q, Wang MY, Shen J, Vallés AS, Lukas RJ, Barrantes FJ, Wu J. The anticonvulsive drug lamotrigine blocks neuronal {alpha}4{beta}2 nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2010; 335:401–408. PMID: 20688974.18. Ahmad S, Fowler LJ, Whitton PS. Lamotrigine, carbamazepine and phenytoin differentially alter extracellular levels of 5-hydroxytryptamine, dopamine and amino acids. Epilepsy Res. 2005; 63:141–149. PMID: 15777732.
Article19. Ahmad S, Fowler LJ, Whitton PS. Effects of combined lamotrigine and valproate on basal and stimulated extracellular amino acids and monoamines in the hippocampus of freely moving rats. Naunyn Schmiedebergs Arch Pharmacol. 2005; 371:1–8. PMID: 15660242.
Article20. Southam E, Kirkby D, Higgins GA, Hagan RM. Lamotrigine inhibits monoamine uptake in vitro and modulates 5-hydroxytryptamine uptake in rats. Eur J Pharmacol. 1998; 358:19–24. PMID: 9809864.
Article21. Reid JG, Gitlin MJ, Altshuler LL. Lamotrigine in psychiatric disorders. J Clin Psychiatry. 2013; 74:675–684. PMID: 23945444.
Article22. Large CH, Webster EL, Goff DC. The potential role of lamotrigine in schizophrenia. Psychopharmacology (Berl). 2005; 181:415–436. PMID: 16001126.
Article23. Li QZ, Cho HS, Jeun SH, Kim KJ, Choi SJ, Sung KW. Effects of grape seed proanthocyanidin on 5-hydroxytryptamine(3) receptors in NCB-20 neuroblastoma cells. Biol Pharm Bull. 2011; 34:1109–1115. PMID: 21720021.
Article24. Yang HS, Kim SY, Choi SJ, Kim KJ, Kim ON, Lee SB, Sung KW. Effect of 5-hydroxyindole on ethanol potentiation of 5-hydroxytryptamine (5-HT)3 receptor-activated ion current in NCB-20 neuroblastoma cells. Neurosci Lett. 2003; 338:72–76. PMID: 12565143.25. Zhou Q, Verdoorn TA, Lovinger DM. Alcohols potentiate the function of 5-HT3 receptor-channels on NCB-20 neuroblastoma cells by favouring and stabilizing the open channel state. J Physiol. 1998; 507:335–352. PMID: 9518697.26. Lovinger DM, Sung KW, Zhou Q. Ethanol and trichloroethanol alter gating of 5-HT3 receptor-channels in NCB-20 neuroblastoma cells. Neuropharmacology. 2000; 39:561–570. PMID: 10728877.27. Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995; 15:181–191. PMID: 7542462.
Article28. Neijt HC, Plomp JJ, Vijverberg HP. Kinetics of the membrane current mediated by serotonin 5-HT3 receptors in cultured mouse neuroblastoma cells. J Physiol. 1989; 411:257–269. PMID: 2482354.29. van Hooft JA, van der Haar E, Vijverberg HP. Allosteric potentiation of the 5-HT3 receptor-mediated ion current in N1E-115 neuroblastoma cells by 5-hydroxyindole and analogues. Neuropharmacology. 1997; 36:649–653. PMID: 9225290.30. Gunthorpe MJ, Lummis SC. Diltiazem causes open channel block of recombinant 5-HT3 receptors. J Physiol. 1999; 519:713–722. PMID: 10457085.31. Maryadale JO, Patricia EH, Peter HD, Kristin JR. The merck index. 15th ed. Cambridge: The Royal Society of Chemistry;2013. p. 5400.32. Choi JS, Choi BH, Ahn HS, Kim MJ, Rhie DJ, Yoon SH, Min DS, Jo YH, Kim MS, Sung KW, Hahn SJ. Mechanism of block by fluoxetine of 5-hydroxytryptamine3 (5-HT3)-mediated currents in NCB-20 neuroblastoma cells. Biochem Pharmacol. 2003; 66:2125–2132. PMID: 14609737.33. Funahashi M, Mitoh Y, Matsuo R. Activation of presynaptic 5-HT3 receptors facilitates glutamatergic synaptic inputs to area postrema neurons in rat brain slices. Methods Find Exp Clin Pharmacol. 2004; 26:615–622. PMID: 15605121.34. Liu W, Thielen RJ, McBride WJ. Effects of repeated daily treatments with a 5-HT3 receptor antagonist on dopamine neurotransmission and functional activity of 5-HT3 receptors within the nucleus accumbens of Wistar rats. Pharmacol Biochem Behav. 2006; 84:370–377. PMID: 16828150.35. Turner TJ, Mokler DJ, Luebke JI. Calcium influx through presynaptic 5-HT3 receptors facilitates GABA release in the hippocampus: in vitro slice and synaptosome studies. Neuroscience. 2004; 129:703–718. PMID: 15541891.36. Lee S, Hjerling-Leffler J, Zagha E, Fishell G, Rudy B. The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J Neurosci. 2010; 30:16796–16808. PMID: 21159951.
Article37. Thompson AJ, Sullivan NL, Lummis SC. Characterization of 5-HT3 receptor mutations identified in schizophrenic patients. J Mol Neurosci. 2006; 30:273–281. PMID: 17401153.
Article38. Thompson AJ, Lummis SC. 5-HT3 receptors. Curr Pharm Des. 2006; 12:3615–3630. PMID: 17073663.39. Xie X, Lancaster B, Peakman T, Garthwaite J. Interaction of the antiepileptic drug lamotrigine with recombinant rat brain type IIA Na+ channels and with native Na+ channels in rat hippocampal neurones. Pflugers Arch. 1995; 430:437–446. PMID: 7491269.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Selective serotonin reuptake inhibitor escitalopram inhibits 5-HT₃ receptor currents in NCB-20 cells
- Tricyclic antidepressant amitriptyline inhibits 5-hydroxytryptamine 3 receptor currents in NCB-20 cells
- Gastroprokinetic agent, mosapride inhibits 5-HT₃ receptor currents in NCB-20 cells
- Haloperidol, a typical antipsychotic, inhibits 5-HT3 receptor- mediated currents in NCB-20 cells: a whole-cell patch-clamp study
- Quetiapine competitively inhibits 5-HT3 receptor-mediated currents in NCB20 neuroblastoma cells