Korean J Physiol Pharmacol.
2018 Sep;22(5):585-595. 10.4196/kjpp.2018.22.5.585.
Tricyclic antidepressant amitriptyline inhibits 5-hydroxytryptamine 3 receptor currents in NCB-20 cells
- Affiliations
-
- 1Department of Anatomy, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea.
- 2Department of Pharmacology, College of Medicine, The Catholic University of Korea, Seoul 06591, Korea. sungkw@catholic.ac.kr
- KMID: 2419008
- DOI: http://doi.org/10.4196/kjpp.2018.22.5.585
Abstract
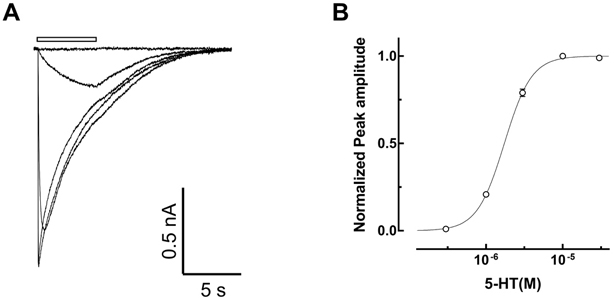
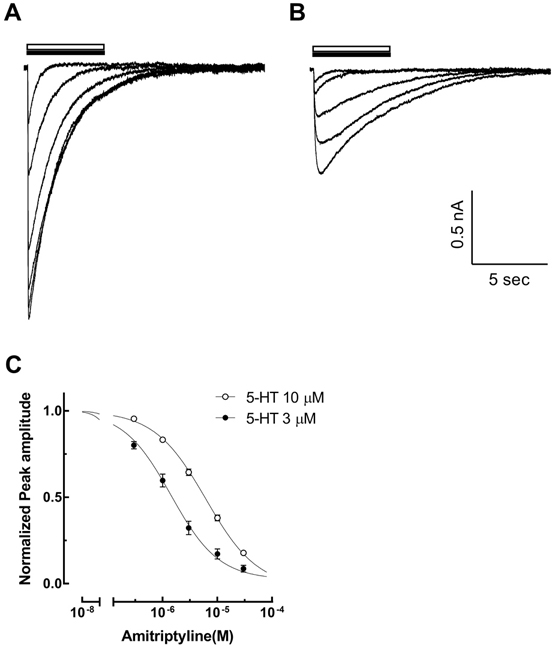
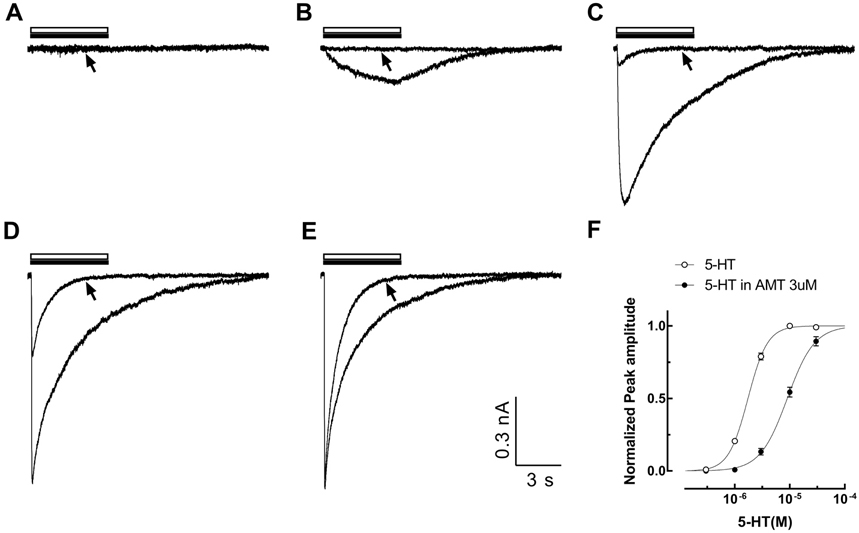
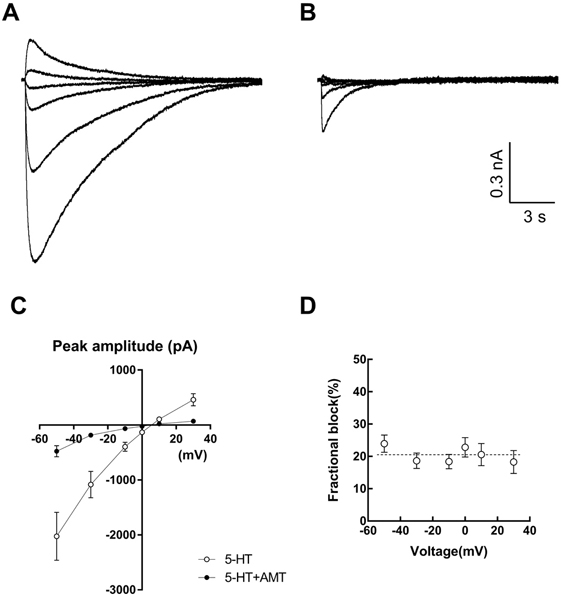
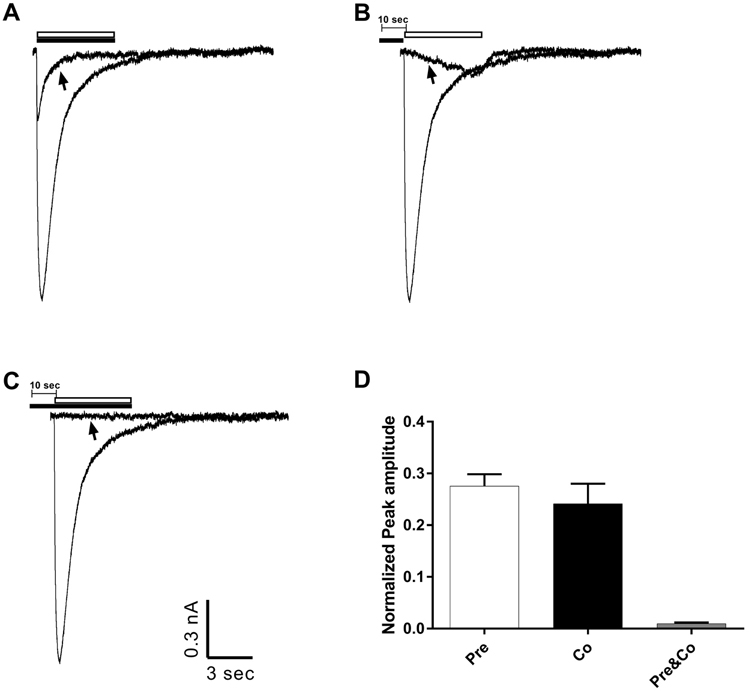
- Amitriptyline, a tricyclic antidepressant, is commonly used to treat depression and neuropathic pain, but its mechanism is still unclear. We tested the effect of amitriptyline on 5-hydroxytryptamine 3 (5-HT₃) receptor currents and studied its blocking mechanism because the clinical applications of amitriptyline overlapped with 5-HT₃ receptor therapeutic potentials. Using a whole-cell voltage clamp method, we recorded the currents of the 5-HT₃ receptor when 5-HT was applied alone or co-applied with amitriptyline in cultured NCB-20 neuroblastoma cells known to express 5-HT₃ receptors. To elucidate the mechanism of amitriptyline, we simulated the 5-HT₃ receptor currents using Berkeley Madonna® software and calculated the rate constants of the agonist binding and receptor transition steps. The 5-HT₃ receptor currents were inhibited by amitriptyline in a concentration-dependent, voltage-independent manner, and a competitive mode. Amitriptyline accelerated the desensitization of the 5-HT₃ receptor. When amitriptyline was applied before 5-HT treatment, the currents rose slowly until the end of 5-HT treatment. When amitriptyline was co-applied with 5-HT, currents rose and decayed rapidly. Peak current amplitudes were decreased in both applications. All macroscopic currents recorded in whole cell voltage clamping experiments were reproduced by simulation and the changes of rate constants by amitriptyline were correlated with macroscopic current recording data. These results suggest that amitriptyline blocks the 5-HT₃ receptor by close and open state blocking mechanisms, in a competitive manner. We could expand an understanding of pharmacological mechanisms of amitriptyline related to the modulation of a 5-HT₃ receptor, a potential target of neurologic and psychiatric diseases through this study.
MeSH Terms
Figure
Cited by 2 articles
-
Gastroprokinetic agent, mosapride inhibits 5-HT3 receptor currents in NCB-20 cells
Yong Soo Park, Ki-Wug Sung
Korean J Physiol Pharmacol. 2019;23(5):419-426. doi: 10.4196/kjpp.2019.23.5.419.Selective serotonin reuptake inhibitor escitalopram inhibits 5-HT3 receptor currents in NCB-20 cells
Yong Soo Park, Ki-Wug Sung
Korean J Physiol Pharmacol. 2019;23(6):509-517. doi: 10.4196/kjpp.2019.23.6.509.
Reference
-
1. Barnes NM, Hales TG, Lummis SC, Peters JA. The 5-HT3 receptor--the relationship between structure and function. Neuropharmacology. 2009; 56:273–284.2. Maricq AV, Peterson AS, Brake AJ, Myers RM, Julius D. Primary structure and functional expression of the 5HT3 receptor, a serotonin-gated ion channel. Science. 1991; 254:432–437.
Article3. Sugita S, Shen KZ, North RA. 5-hydroxytryptamine is a fast excitatory transmitter at 5-HT3 receptors in rat amygdala. Neuron. 1992; 8:199–203.
Article4. Katsurabayashi S, Kubota H, Tokutomi N, Akaike N. A distinct distribution of functional presynaptic 5-HT receptor subtypes on GABAergic nerve terminals projecting to single hippocampal CA1 pyramidal neurons. Neuropharmacology. 2003; 44:1022–1030.
Article5. Thompson AJ, Lummis SC. 5-HT3 receptors. Curr Pharm Des. 2006; 12:3615–3630.
Article6. Thompson AJ, Lummis SC. The 5-HT3 receptor as a therapeutic target. Expert Opin Ther Targets. 2007; 11:527–540.7. Navari RM. 5-HT3 receptors as important mediators of nausea and vomiting due to chemotherapy. Biochim Biophys Acta. 2015; 1848:2738–2746.
Article8. De Deurwaerdère P, Moison D, Navailles S, Porras G, Spampinato U. Regionally and functionally distinct serotonin3 receptors control in vivo dopamine outflow in the rat nucleus accumbens. J Neurochem. 2005; 94:140–149.
Article9. Campbell AD, Kohl RR, McBride WJ. Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol. 1996; 13:569–574.
Article10. Feinberg-Zadek PL, Davies PA. Ethanol stabilizes the open state of single 5-hydroxytryptamine(3A)(QDA) receptors. J Pharmacol Exp Ther. 2010; 333:896–902.
Article11. Lovinger DM, Sung KW, Zhou Q. Ethanol and trichloroethanol alter gating of 5-HT3 receptor-channels in NCB-20 neuroblastoma cells. Neuropharmacology. 2000; 39:561–570.
Article12. Lummis SC. 5-HT(3) receptors. J Biol Chem. 2012; 287:40239–40245.
Article13. Wilde MI, Markham A. Ondansetron. A review of its pharmacology and preliminary clinical findings in novel applications. Drugs. 1996; 52:773–794.14. Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997; 283:1305–1322.15. Galletti F, Cupini LM, Corbelli I, Calabresi P, Sarchielli P. Pathophysiological basis of migraine prophylaxis. Prog Neurobiol. 2009; 89:176–192.
Article16. Kremer M, Salvat E, Muller A, Yalcin I, Barrot M. Antidepressants and gabapentinoids in neuropathic pain: Mechanistic insights. Neuroscience. 2016; 338:183–206.
Article17. Morgan V, Pickens D, Gautam S, Kessler R, Mertz H. Amitriptyline reduces rectal pain related activation of the anterior cingulate cortex in patients with irritable bowel syndrome. Gut. 2005; 54:601–607.
Article18. Gumilar F, Bouzat C. Tricyclic antidepressants inhibit homomeric Cys-loop receptors by acting at different conformational states. Eur J Pharmacol. 2008; 584:30–39.
Article19. Shimizu M, Nishida A, Zensho H, Yamawaki S. Chronic amitripty-line exposure reduces 5-HT3 receptor-mediated cyclic GMP formation in NG 108-15 cells. Brain Res. 1996; 741:89–94.
Article20. Lovinger DM, White G. Ethanol potentiation of 5-hydroxytryptamine3 receptor-mediated ion current in neuroblastoma cells and isolated adult mammalian neurons. Mol Pharmacol. 1991; 40:263–270.21. Choi JS, Choi BH, Ahn HS, Kim MJ, Rhie DJ, Yoon SH, Min DS, Jo YH, Kim MS, Sung KW, Hahn SJ. Mechanism of block by fluoxetine of 5-hydroxytryptamine3 (5-HT3)-mediated currents in NCB-20 neuroblastoma cells. Biochem Pharmacol. 2003; 66:2125–2132.
Article22. Li QZ, Cho HS, Jeun SH, Kim KJ, Choi SJ, Sung KW. Effects of grape seed proanthocyanidin on 5-hydroxytryptamine(3) receptors in NCB-20 neuroblastoma cells. Biol Pharm Bull. 2011; 34:1109–1115.
Article23. Kim KJ, Jeun SH, Sung KW. Lamotrigine, an antiepileptic drug, inhibits 5-HT3 receptor currents in NCB-20 neuroblastoma cells. Korean J Physiol Pharmacol. 2017; 21:169–177.24. Lambert JJ, Peters JA, Hales TG, Dempster J. The properties of 5-HT3 receptors in clonal cell lines studied by patch-clamp techniques. Br J Pharmacol. 1989; 97:27–40.
Article25. Alberola-Die A, Martinez-Pinna J, González-Ros JM, Ivorra I, Morales A. Multiple inhibitory actions of lidocaine on Torpedo nicotinic acetylcholine receptors transplanted to Xenopus oocytes. J Neurochem. 2011; 117:1009–1019.
Article26. Föhr KJ, Zeller K, Georgieff M, Köster S, Adolph O. Open channel block of NMDA receptors by diphenhydramine. Neuropharmacology. 2015; 99:459–470.
Article27. Gunthorpe MJ, Lummis SC. Diltiazem causes open channel block of recombinant 5-HT3 receptors. J Physiol. 1999; 519:713–722.28. Hapfelmeier G, Tredt C, Haseneder R, Zieglgänsberger W, Eisensamer B, Rupprecht R, Rammes G. Co-expression of the 5-HT3B serotonin receptor subunit alters the biophysics of the 5-HT3 receptor. Biophys J. 2003; 84:1720–1733.
Article29. Mott DD, Erreger K, Banke TG, Traynelis SF. Open probability of homomeric murine 5-HT3A serotonin receptors depends on subunit occupancy. J Physiol. 2001; 535:427–443.30. Zhou Q, Verdoorn TA, Lovinger DM. Alcohols potentiate the function of 5-HT3 receptor-channels on NCB-20 neuroblastoma cells by favouring and stabilizing the open channel state. J Physiol. 1998; 507:335–352.31. Zheng C, Yang K, Liu Q, Wang MY, Shen J, Vallés AS, Lukas RJ, Barrantes FJ, Wu J. The anticonvulsive drug lamotrigine blocks neuronal {alpha}4{beta}2 nicotinic acetylcholine receptors. J Pharmacol Exp Ther. 2010; 335:401–408.32. Thompson AJ, Jarvis GE, Duke RK, Johnston GA, Lummis SC. Ginkgolide B and bilobalide block the pore of the 5-HT3receptor at a location that overlaps the picrotoxin binding site. Neuropharmacology. 2011; 60:488–495.33. Buéno L, Fioramonti J, Garcia-Villar R. Pathobiology of visceral pain: molecular mechanisms and therapeutic implications. III. Visceral afferent pathways: a source of new therapeutic targets for abdominal pain. Am J Physiol Gastrointest Liver Physiol. 2000; 278:G670–G676.34. Hornung JP. The human raphe nuclei and the serotonergic system. J Chem Neuroanat. 2003; 26:331–343.
Article35. Hayashida K, Kimura M, Yoshizumi M, Hobo S, Obata H, Eisenach JC. Ondansetron reverses antihypersensitivity from clonidine in rats after peripheral nerve injury: role of γ-aminobutyric acid in α2-adrenoceptor and 5-HT3 serotonin receptor analgesia. Anesthesiology. 2012; 117:389–398.36. Oatway MA, Chen Y, Weaver LC. The 5-HT3 receptor facilitates at-level mechanical allodynia following spinal cord injury. Pain. 2004; 110:259–268.
Article37. Saria A, Javorsky F, Humpel C, Gamse R. 5-HT3 receptor antagonists inhibit sensory neuropeptide release from the rat spinal cord. Neuroreport. 1990; 1:104–106.
Article38. Zeitz KP, Guy N, Malmberg AB, Dirajlal S, Martin WJ, Sun L, Bonhaus DW, Stucky CL, Julius D, Basbaum AI. The 5-HT3 subtype of serotonin receptor contributes to nociceptive processing via a novel subset of myelinated and unmyelinated nociceptors. J Neurosci. 2002; 22:1010–1019.39. Guo W, Miyoshi K, Dubner R, Gu M, Li M, Liu J, Yang J, Zou S, Ren K, Noguchi K, Wei F. Spinal 5-HT3 receptors mediate descending facilitation and contribute to behavioral hypersensitivity via a reciprocal neuron-glial signaling cascade. Mol Pain. 2014; 10:35.
Article40. Maizels M, McCarberg B. Antidepressants and antiepileptic drugs for chronic non-cancer pain. Am Fam Physician. 2005; 71:483–490.41. Rajkumar R, Mahesh R. The auspicious role of the 5-HT3 receptor in depression: a probable neuronal target? J Psychopharmacol. 2010; 24:455–469.42. Eisensamer B, Rammes G, Gimpl G, Shapa M, Ferrari U, Hapfelmeier G, Bondy B, Parsons C, Gilling K, Zieglgänsberger W, Holsboer F, Rupprecht R. Antidepressants are functional antagonists at the serotonin type 3 (5-HT3) receptor. Mol Psychiatry. 2003; 8:994–1007.
Article43. Gupta D, Radhakrishnan M, Kurhe Y. 5HT3 receptor antagonist (ondansetron) reverses depressive behavior evoked by chronic unpredictable stress in mice: modulation of hypothalamic-pituitary-adrenocortical and brain serotonergic system. Pharmacol Biochem Behav. 2014; 124:129–136.
Article44. Gupta D, Radhakrishnan M, Kurhe Y, Thangaraj D, Prabhakar V, Kanade P. Antidepressant-like effects of a novel 5-HT3 receptor antagonist 6z in acute and chronic murine models of depression. Acta Pharmacol Sin. 2014; 35:1493–1503.
Article45. Johnson BA, Ait-Daoud N, Ma JZ, Wang Y. Ondansetron reduces mood disturbance among biologically predisposed, alcohol-dependent individuals. Alcohol Clin Exp Res. 2003; 27:1773–1779.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lamotrigine, an antiepileptic drug, inhibits 5-HT₃ receptor currents in NCB-20 neuroblastoma cells
- Gastroprokinetic agent, mosapride inhibits 5-HT₃ receptor currents in NCB-20 cells
- Selective serotonin reuptake inhibitor escitalopram inhibits 5-HT₃ receptor currents in NCB-20 cells
- Resting Tremor during Low-dose Tricyclic Antidepressant Treatment: A case report
- A Clinical Study on the Treatment of Postherpetic Neuralgia