Ann Lab Med.
2017 May;37(3):277-281. 10.3343/alm.2017.37.3.277.
Clinical Usefulness of Monitoring Cytomegalovirus-Specific Immunity by Quantiferon-CMV in Pediatric Allogeneic Hematopoietic Stem Cell Transplantation Recipients
- Affiliations
-
- 1Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. eskang@skku.edu
- 2Stem Cell & Regenerative Medicine Institute, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2369755
- DOI: http://doi.org/10.3343/alm.2017.37.3.277
Abstract
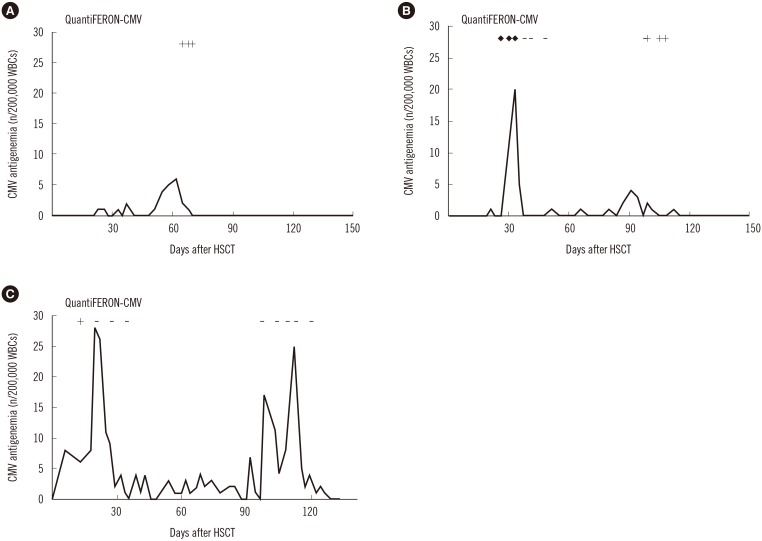
- Cytomegalovirus (CMV) is a well-established cause of morbidity and mortality in pediatric recipients of allogeneic hematopoietic stem cell transplantation (allo-HSCT). CD8⺠T-cells are important for controlling CMV infection. We conducted a prospective pilot study to investigate the clinical utility of measuring the CMV-specific T-cell immune response using the QuantiFERON-CMV assay (QF-CMV) in pediatric allo-HSCT recipients. Overall, 16 of 25 (64%) patients developed CMV infection. QF-CMV was evaluated in these 16 patients during the early and late phases of the first CMV infection post allo-HSCT. Whereas the initial QF-CMV results during the early phase of CMV infection did not correlate with the course of the corresponding infection, the QF-CMV results post resolution of the first CMV infection correlated with the recurrence of CMV infection until 12 months post allo-HSCT; no recurrent infections occurred in the four QF-CMV-positive patients, while recurrent infections manifested in five of eight QF-CMV-negative (62.5%) and all three QF-CMV-indeterminate patients (P=0.019). In spite of the small number of patients examined, this study supports the potential application of monitoring CMV-specific T-cell immunity using the QF-CMV assay to predict the recurrence of CMV infection in pediatric allo-HSCT recipients.
Keyword
MeSH Terms
Figure
Reference
-
1. Hebart H, Einsele H. Clinical aspects of CMV infection after stem cell transplantation. Hum Immunol. 2004; 65:432–436. PMID: 15172442.2. Castagnola E, Cappelli B, Erba D, Rabagliati A, Lanino E, Dini G. Cytomegalovirus infection after bone marrow transplantation in children. Hum Immunol. 2004; 65:416–422. PMID: 15172440.3. Ljungman P. CMV infections after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008; 42(Sl):S70–S72. PMID: 18724309.4. Meijer E, Boland GJ, Verdonck LF. Prevention of cytomegalovirus disease in recipients of allogeneic stem cell transplants. Clin Microbiol Rev. 2003; 16:647–657. PMID: 14557291.5. Goodrich JM, Bowden RA, Fisher L, Keller C, Schoch G, Meyers JD. Ganciclovir prophylaxis to prevent cytomegalovirus disease after allogeneic marrow transplant. Ann Intern Med. 1993; 118:173–178. PMID: 8380242.6. Goodrich JM, Boeckh M, Bowden R. Strategies for the prevention of cytomegalovirus disease after marrow transplantation. Clin Infect Dis. 1994; 19:287–298. PMID: 7986901.7. Boeckh M, Fries B, Nichols WG. Recent advances in the prevention of CMV infection and disease after hematopoietic stem cell transplantation. Pediatr Transplant. 2004; 8(S5):S19–S27.8. Crough T, Khanna R. Immunobiology of human cytomegalovirus: from bench to bedside. Clin Microbiol Rev. 2009; 22:76–98. PMID: 19136435.9. Harari A, Zimmerli SC, Pantaleo G. Cytomegalovirus (CMV)-specific cellular immune responses. Hum Immunol. 2004; 65:500–506. PMID: 15172450.10. Avetisyan G, Aschan J, Hägglund H, Ringdén O, Ljungman P. Evaluation of intervention strategy based on CMV-specific immune responses after allogeneic SCT. Bone Marrow Transplant. 2007; 40:865–869. PMID: 17724444.11. Walker S, Fazou C, Crough T, Holdsworth R, Kiely P, Veale M, et al. Ex vivo monitoring of human cytomegalovirus-specific CD8+ T-cell responses using QuantiFERON-CMV. Transpl Infect Dis. 2007; 9:165–170. PMID: 17462006.12. Tey SK, Kennedy GA, Cromer D, Davenport MP, Walker S, Jones LI, et al. Clinical assessment of anti-viral CD8+ T cell immune monitoring using QuantiFERON-CMV® assay to identify high risk allogeneic hematopoietic stem cell transplant patients with CMV infection complications. PLoS One. 2013; 8:e74744. PMID: 24146744.13. Manuel O, Husain S, Kumar D, Zayas C, Mawhorter S, Levi ME, et al. Assessment of cytomegalovirus-specific cell-mediated immunity for the prediction of cytomegalovirus disease in high-risk solid-organ transplant recipients: a multicenter cohort study. Clin Infect Dis. 2013; 56:817–824. PMID: 23196955.14. Lochmanova A, Lochman I, Tomaskova H, Marsalkova P, Raszka J, Mrazek J, et al. Quantiferon-CMV test in prediction of cytomegalovirus infection after kidney transplantation. Transplant Proc. 2010; 42:3574–3577. PMID: 21094818.15. Fleming T, Dunne J, Crowley B. Ex vivo monitoring of human cytomegalovirus-specific CD8(+) T-Cell responses using the QuantiFERON-CMV assay in allogeneic hematopoietic stem cell transplant recipients attending an Irish hospital. J Med Virol. 2010; 82:433–440. PMID: 20087937.16. Kumar D, Chernenko S, Moussa G, Cobos I, Manuel O, Preiksaitis J, et al. Cell-mediated immunity to predict cytomegalovirus disease in high-risk solid organ transplant recipients. Am J Transplant. 2009; 9:1214–1222. PMID: 19422346.17. Abate D, Cesaro S, Cofano S, Fiscon M, Saldan A, Varotto S, et al. Diagnostic utility of human cytomegalovirus-specific T-cell response monitoring in predicting viremia in pediatric allogeneic stem-cell transplant patients. Transplantation. 2012; 93:536–542. PMID: 22314338.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Primary Cytomegalovirus Peritonitis Following Unrelated Hematopoietic Stem Cell Transplantation
- Comparison of Quality of Life of Autologous and Allogeneic Hematopoietic Stem Cell Transplantation Recipients
- Immunological Prediction of Cytomegalovirus (CMV) Replication Risk in Solid Organ Transplantation Recipients: Approaches for Regulating the Targeted Anti-CMV Prevention Strategies
- Comparison of the Commercial QuantiFERON-CMV and Overlapping Peptide-based ELISPOT Assays for Predicting CMV Infection in Kidney Transplant Recipients
- Cytomegalovirus Infection according to Cell Source after Hematopoietic Cell Transplantation in Pediatric Patients