Ann Dermatol.
2017 Feb;29(1):1-5. 10.5021/ad.2017.29.1.1.
Effectiveness of Specific Sublingual Immunotherapy in Korean Patients with Atopic Dermatitis
- Affiliations
-
- 1Department of Dermatology, Pusan National University School of Medicine, Busan, Korea. dockbs@pusan.ac.kr
- 2Biomedical Research Institute, Pusan National University Hospital, Busan, Korea.
- KMID: 2368020
- DOI: http://doi.org/10.5021/ad.2017.29.1.1
Abstract
- BACKGROUND
Sublingual immunotherapy (SLIT) with house dust mites (HDM) preparation has recently been proven to be beneficial for treating allergic rhinitis and asthma. However, there has been no report regarding the efficacy and safety of SLIT in Korean patients with atopic dermatitis (AD).
OBJECTIVE
We intended to investigate the efficacy and safety of SLIT in Korean patients with AD.
METHODS
A total of 34 patients with AD and immunoglobulin E (IgE)-proven HDM sensitization (Class ≥3) were recruited. Eczema area and severity index (EASI) score, total serum IgE level, specific IgE assays to Dermatophagoides pteronyssinus, D. farinae, and adverse effects were recorded during follow-up. "Responder" was defined as a patient with ≥30% improvement in EASI score after SLIT.
RESULTS
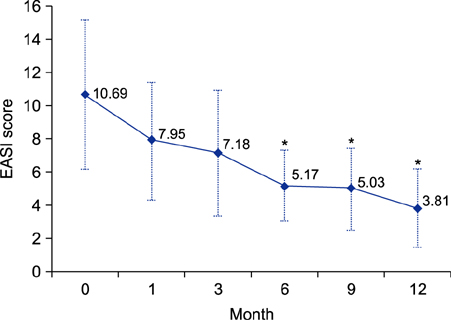
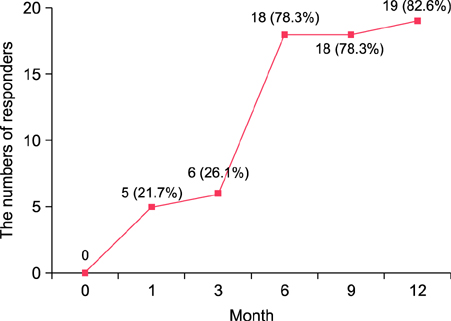
Twenty-three patients continued SLIT for 12 months or more, whereas 3 patients (8.8%) dropped out because of exacerbation of dermatitis, and 8 patients (23.5%) were lost to follow-up. The average duration of SLIT treatment was 22.4 months (range, 12~32 months). EASI scores reduced significantly after 6 months of treatment (p<0.05) compared with those at baseline. A total of 18 patients were determined to be responders to SLIT after 6 months. Total and specific IgE serum levels did not significantly reduce after SLIT. No patients experienced serious adverse events, with the exception of two patients who developed transient lip and tongue swelling.
CONCLUSION
Our study demonstrated that SLIT with HDM extracts is effective and tolerable in Korean patients with AD. Further controlled long-term trials are required to reinforce the current results.
MeSH Terms
Figure
Reference
-
1. Pajno GB. Sublingual immunotherapy: the optimism and the issues. J Allergy Clin Immunol. 2007; 119:796–801.2. Bae JM, Choi YY, Park CO, Chung KY, Lee KH. Efficacy of allergen-specific immunotherapy for atopic dermatitis: a systematic review and meta-analysis of randomized controlled trials. J Allergy Clin Immunol. 2013; 132:110–117.
Article3. Qin YE, Mao JR, Sang YC, Li WX. Clinical efficacy and compliance of sublingual immunotherapy with Dermatophagoides farinae drops in patients with atopic dermatitis. Int J Dermatol. 2014; 53:650–655.
Article4. Cadario G, Galluccio AG, Pezza M, Appino A, Milani M, Pecora S, et al. Sublingual immunotherapy efficacy in patients with atopic dermatitis and house dust mites sensitivity: a prospective pilot study. Curr Med Res Opin. 2007; 23:2503–2506.
Article5. Silny W, Czarnecka-Operacz M. Specific immunotherapy in the treatment of patients with atopic dermatitis--results of double blind placebo controlled study. Pol Merkur Lekarski. 2006; 21:558–565.6. Bieber T. Atopic dermatitis. N Engl J Med. 2008; 358:1483–1494.7. Vanbervliet B, Tourdot S, Mascarell L, Rouzaire P, Vocanson M, Rozières A, et al. SLIT prevents the development of eczema in percutaneous allergen-sensitized mice. J Invest Dermatol. 2012; 132:244–246.
Article8. Passalacqua G, Compalati E, Canonica GW. Sublingual immunotherapy: clinical indications in the WAO-SLIT position paper. World Allergy Organ J. 2010; 3:216–219.
Article9. Di Rienzo V, Cadario G, Grieco T, Galluccio AG, Caffarelli C, Liotta G, et al. Sublingual immunotherapy in mite-sensitized children with atopic dermatitis: a randomized, open, parallel-group study. Ann Allergy Asthma Immunol. 2014; 113:671–673.e1.
Article10. Pajno GB, Caminiti L, Vita D, Barberio G, Salzano G, Lombardo F, et al. Sublingual immunotherapy in mite-sensitized children with atopic dermatitis: a randomized, double-blind, placebo-controlled study. J Allergy Clin Immunol. 2007; 120:164–170.
Article11. Novak N. Allergen specific immunotherapy for atopic dermatitis. Curr Opin Allergy Clin Immunol. 2007; 7:542–546.
Article12. Werfel T, Breuer K, Ruéff F, Przybilla B, Worm M, Grewe M, et al. Usefulness of specific immunotherapy in patients with atopic dermatitis and allergic sensitization to house dust mites: a multi-centre, randomized, dose-response study. Allergy. 2006; 61:202–205.
Article13. Canonica GW, Bousquet J, Casale T, Lockey RF, Baena-Cagnani CE, Pawankar R, et al. Sub-lingual immunotherapy: world allergy organization position paper 2009. World Allergy Organ J. 2009; 2:233–281.14. Cox LS, Larenas Linnemann D, Nolte H, Weldon D, Finegold I, Nelson HS. Sublingual immunotherapy: a comprehensive review. J Allergy Clin Immunol. 2006; 117:1021–1035.
Article15. Gómez Vera J, Flores Sandoval G, Orea Solano M, López Tiro J, Jiménez Saab N. Safety and efficacy of specific sublingual immunotherapy in patients with asthma and allergy to Dermatophagoides pteronyssinus. Rev Alerg Mex. 2005; 52:231–236.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment of Patients with Refractory Atopic Dermatitis Sensitized to House Dust Mites by Using Sublingual Allergen Immunotherapy
- Two Cases of Atopic Dermatitis Improved by Combination Treatment of Allergen-Specific Immunotherapy and Histamine-Immunoglobulin Complex
- Allergen-specific Immunotherapy in Patients with Atopic Dermatitis
- Dermatophagoides Farinae-specific IgE and IgG4 Antibodies in Atopic Dermatitis Patients
- Nipple Involvement in Atopic Dermatitis: Report of 3 cases