J Bacteriol Virol.
2016 Dec;46(4):193-200. 10.4167/jbv.2016.46.4.193.
Invasion of Mammalian Cells by Rough Variant of Mycobacterium abscessus
- Affiliations
-
- 1Department of Microbiology, and Biomedical Sciences, College of Medicine, Chungnam National University, Daejeon, Korea. hjukim@cnu.ac.kr
- KMID: 2366864
- DOI: http://doi.org/10.4167/jbv.2016.46.4.193
Abstract
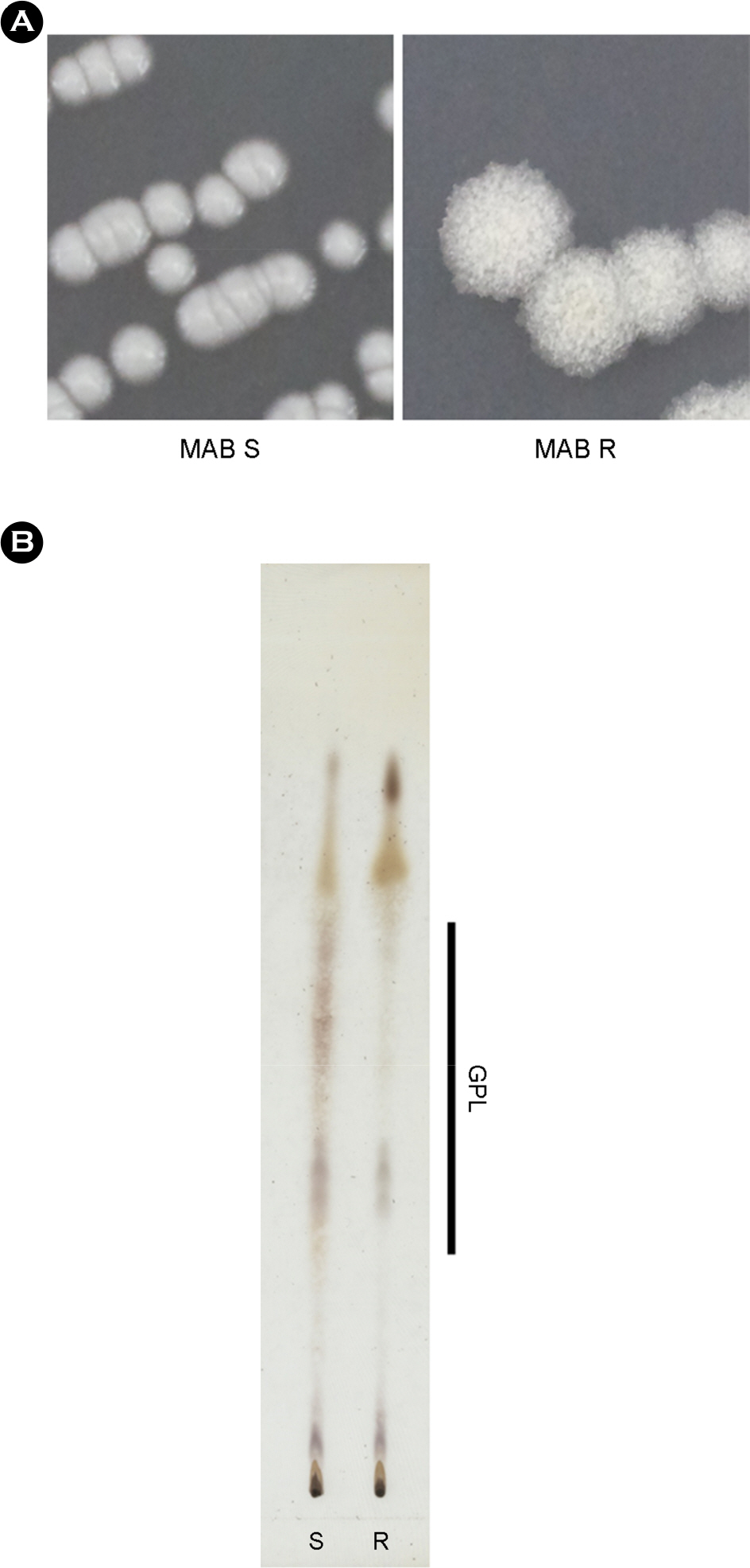
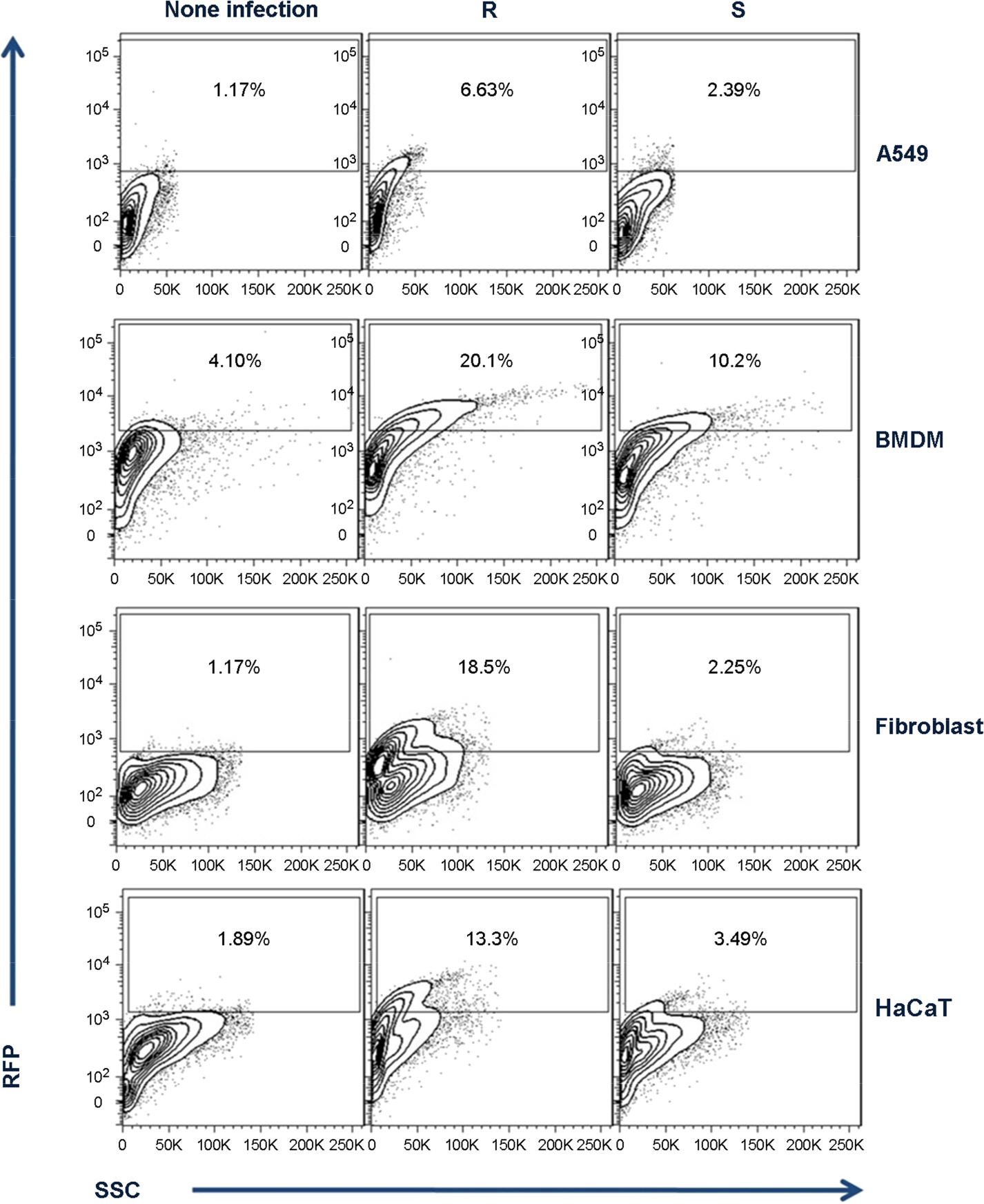
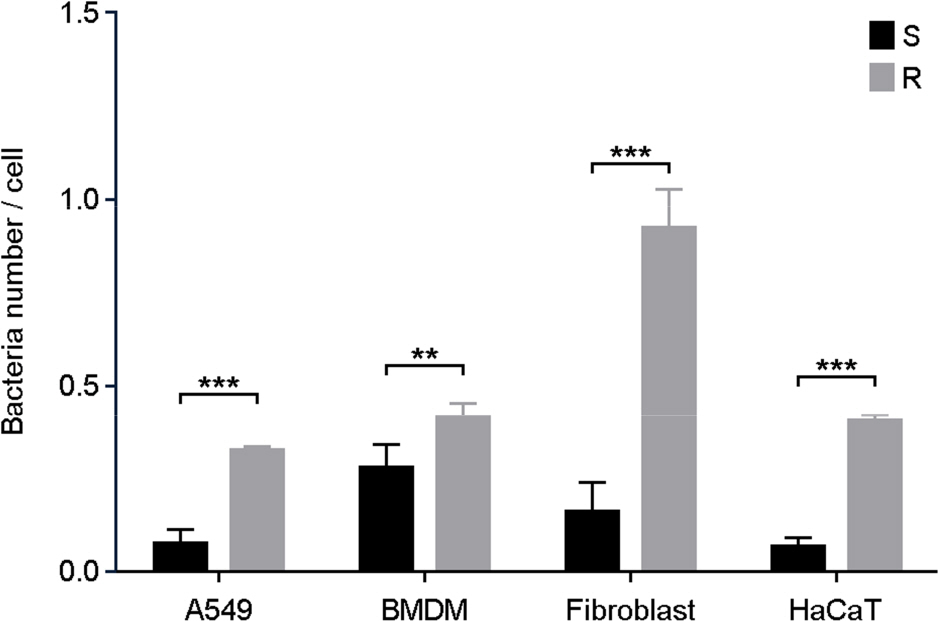
- One of a rapid growing mycobacteria (RGM), Mycobacterium abscessus (MAB), is the most causative agents of RGM pulmonary disease. MAB can change their morphology that smooth (S) type to more virulent type of rough (R). Bacterial invasion into host cells is an important first step to initiate their infection. The phagocytic and invasion mechanisms of Mycobacterium tuberculosis through the host-parasite interaction have been researched. Although MAB causes a wide range of clinical diseases, little is known about their invasion ability or why the R type is more virulent than the S type. To compare invasion ability of R with S types, their infection abilities to dermal fibroblast, HaCaT cells, A549 cells and bone marrow derived macrophages were analyzed. After 2 h of infection, intracellular survival numbers of the R type were significantly higher in all infected cells than S types. The fluorescence-activated cell sorting (FACS) and confocal microscopy assay also revealed that red fluorescent amount and intracellular bacterial numbers in all of the cells infected with MAB R type expressing the red fluorescent protein (RFP) were significantly higher than the S type. Our data suggest that the virulence of MAB is proportionally related to the invasion ability into mammalian cells and macrophages.
MeSH Terms
Figure
Reference
-
1). Griffith DE, Brown-Elliott BA, Benwill JL, Wallace RJ Jr. Mycobacterium abscessus. "Pleased to meet you, hope you guess my name". Ann Am Thorac Soc. 2015; 12:436–9.2). Gomez-Smith CK, LaPara TM, Hozalski RM. Sulfate reducing bacteria and mycobacteria dominate the biofilm communities in a chloraminated drinking water distribution system. Environ Sci Technol. 2015; 49:8432–40.
Article3). Koh WJ, Kwon OJ, Lee KS. Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci. 2005; 20:913–25.
Article4). Kim SY, Shin SJ, Jeong BH, Koh WJ. Successful antibiotic treatment of pulmonary disease caused by Mycobacterium abscessus subsp. abscessus with C-to-T mutation at posi tion 19 in erm(41) gene: case report. BMC Infect Dis. 2016; 16:207.
Article5). Harris KA, Kenna DT. Mycobacterium abscessus infection in cystic fibrosis: molecular typing and clinical outcomes. J Med Microbiol. 2014; 63:1241–6.6). Roux AL, Catherinot E, Soismier N, Heym B, Bellis G, Lemonnier L, et al. Comparing Mycobacterium massiliense and Mycobacterium abscessus lung infections in cystic fibrosis patients. J Cyst Fibros. 2015; 14:63–9.7). Yin L, Yao Y, Guan HJ, Zhang JF, Shi HH, Yang L. Pathological study of Mycobacterium abscessus keratitis. Zhonghua Yan Ke Za Zhi. 2010; 46:829–33.8). Choi GE, Shin SJ, Won CJ, Min KN, Oh T, Hahn MY, et al. Macrolide treatment for Mycobacterium abscessus and Mycobacterium massiliense infection and inducible resistance. Am J Respir Crit Care Med. 2012; 186:917–25.9). Lee SH, Yoo HK, Kim SH, Koh WJ, Kim CK, Park YK, et al. The drug resistance profile of Mycobacterium abscessus group strains from Korea. Ann Lab Med. 2014; 34:31–7.10). Belisle JT, Klaczkiewicz K, Brennan PJ, Jacobs WR Jr, Inamine JM. Rough morphological variants of Mycobacterium avium. Characterization of genomic deletions resulting in the loss of glycopeptidolipid expression. J Biol Chem. 1993; 268:10517–23.11). Pawlik A, Garnier G, Orgeur M, Tong P, Lohan A, Le Chevalier F, et al. Identification and characterization of the genetic changes responsible for the characteristic smooth-to-rough morphotype alterations of clinically persistent Mycobacterium abscessus. Mol Microbiol. 2013; 90:612–29.12). Howard ST, Rhoades E, Recht J, Pang X, Alsup A, Kolter R, et al. Spontaneous reversion of Mycobacterium abscessus from a smooth to a rough morphotype is associated with reduced expression of glycopeptidolipid and reacquisition of an invasive phenotype. Microbiology. 2006; 152:1581–90.13). Koh WJ, Kwon OJ, Jeon K, Kim TS, Lee KS, Park YK, et al. Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest. 2006; 129:341–8.
Article14). Sohn H, Kim HJ, Kim JM, Jung KO, Koh WJ, Shin SJ. High virulent clinical isolates of Mycobacterium abscessus from patients with the upper lobe fibrocavitary form of pulmonary disease. Microb Pathog. 2009; 47:321–8.15). Ribet D, Cossart P. How bacterial pathogens colonize their hosts and invade deeper tissues. Microbes Infect. 2015; 17:173–83.
Article16). Everman JL, Bermudez LE. Antibodies against invasive phenotype-specific antigens increase Mycobacterium avium subspecies paratuberculosis translocation across a polarized epithelial cell model and enhance killing by bovine macrophages. Front Cell Infect Microbiol. 2015; 5:58.17). Saini NK, Sinha R, Singh P, Sharma M, Pathak R, Rathor N, et al. Mce4A protein of Mycobacterium tuberculosis induces pro inflammatory cytokine response leading to macrophage apoptosis in a TNF-alpha dependent manner. Microb Pathog. 2016; 100:43–50.18). Kim SY, Sohn H, Choi GE, Cho SN, Oh T, Kim HJ, et al. Conversion of Mycobacterium smegmatis to a pathogenic phenotype via passage of epithelial cells during macrophage infection. Med Microbiol Immunol. 2011; 200:177–91.19). Sohn H, Kim K, Lee KS, Choi HG, Lee KI, Shin AR, et al. Lithium inhibits growth of intracellular Mycobacterium kansasii through enhancement of macrophage apoptosis. J Microbiol. 2014; 52:299–306.20). Choi GE, Min KN, Won CJ, Jeon K, Shin SJ, Koh WJ. Activities of moxifloxacin in combination with macrolides against clinical isolates of Mycobacterium abscessus and Mycobacterium massiliense. Antimicrob Agents Chemother. 2012; 56:3549–55.21). Choi GE, Chang CL, Whang J, Kim HJ, Kwon OJ, Koh WJ, et al. Efficient differentiation of Mycobacterium abscessus complex isolates to the species level by a novel PCR-based variable-number tandem-repeat assay. J Clin Microbiol. 2011; 49:1107–9.22). Sohn H, Kim KW, Kang HB, Won CJ, Kim WS, Lee B, et al. Induction of macrophage death by clinical strains of Mycobacterium kansasii. Microb Pathog. 2010; 48:160–7.23). Rhoades ER, Archambault AS, Greendyke R, Hsu FF, Streeter C, Byrd TF. Mycobacterium abscessus glycopeptidolipids mask underlying cell wall phosphatidyl-myo-inositol mannosides blocking induction of human macrophage TNF-alpha by preventing interaction with TLR2. J Immunol. 2009; 183:1997–2007.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Autophagy in Mycobacterium abscessus Infection
- Cytokine Profiles of Macrophages by Mycobacterium abscessus Conditional Morphotype Variants and Comparison of Their Extracellular-Protein Expressions
- Two Cases of Cutaneous Mycobacterium abscessus Infection after Mesotherapy
- Current Understanding of Mycobacterium abscessus Infection
- Cutaneous Infection Due to Mycobacterium abscessus on the Buttock