Yonsei Med J.
2015 Jul;56(4):1087-1096. 10.3349/ymj.2015.56.4.1087.
Correlation between Fluorescein Angiographic Findings and Visual Acuity in Behcet Retinal Vasculitis
- Affiliations
-
- 1Department of Ophthalmology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 2Institute of Vision Research, Department of Ophthalmology, Severance Eye and ENT Hospital, Yonsei University College of Medicine, Seoul, Korea. sunglee@yuhs.ac
- KMID: 2366352
- DOI: http://doi.org/10.3349/ymj.2015.56.4.1087
Abstract
- PURPOSE
To identify significant fluorescein angiographic (FA) characteristics associated with visual acuity (VA) in Behcet retinal vasculitis.
MATERIALS AND METHODS
Retrospective review of 86 eyes of 48 patients (age: 35.6+/-10.2 years) with Behcet retinal vasculitis were performed. VA and FA findings as well as correlation between them were assessed.
RESULTS
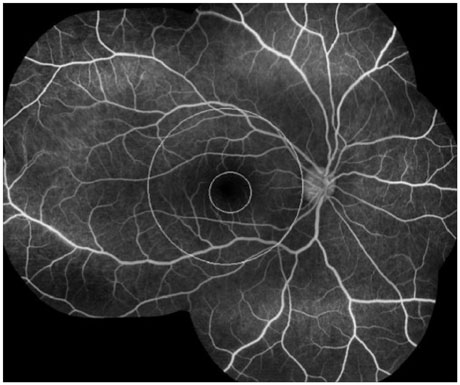
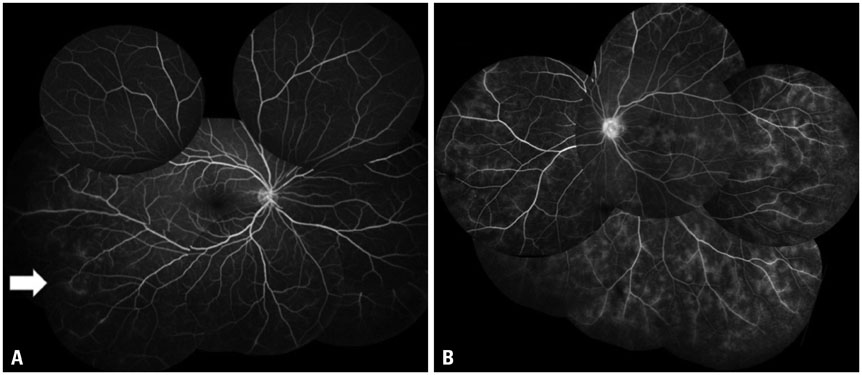
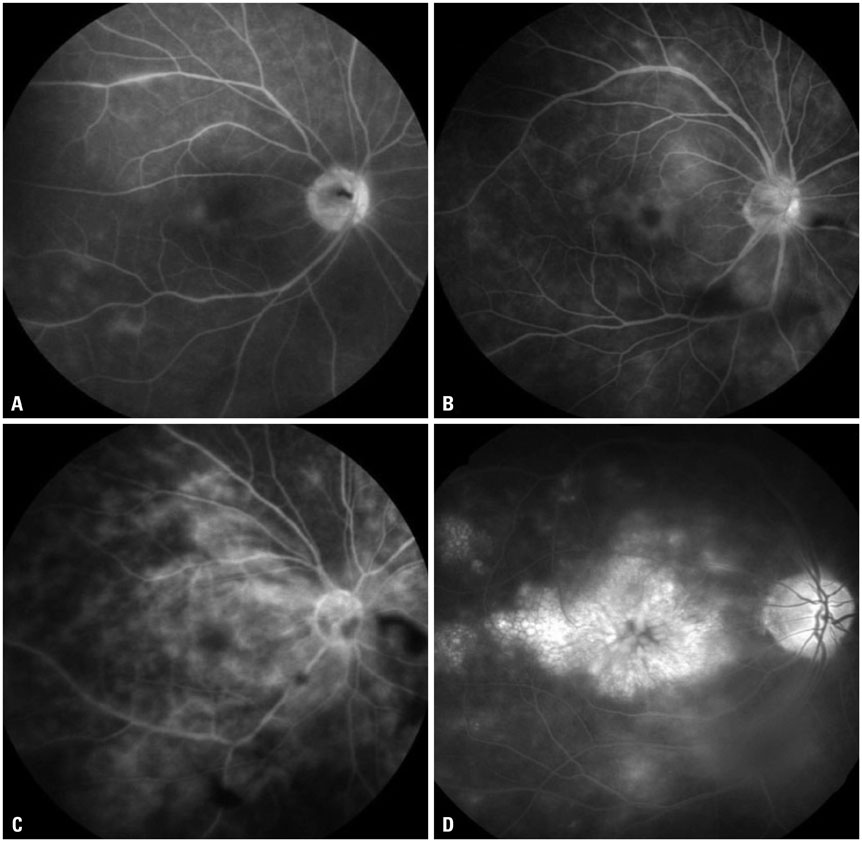
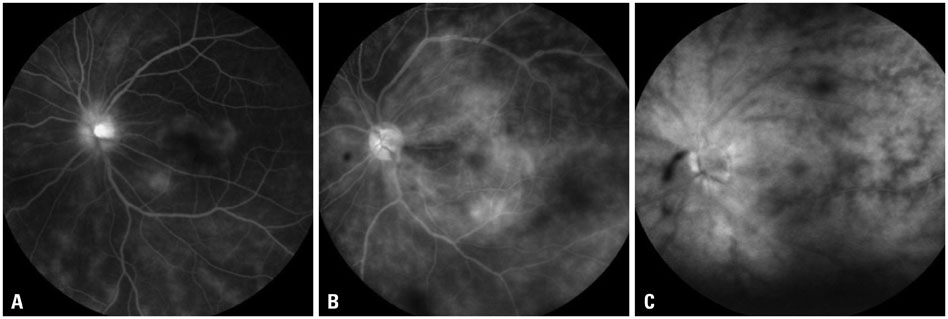
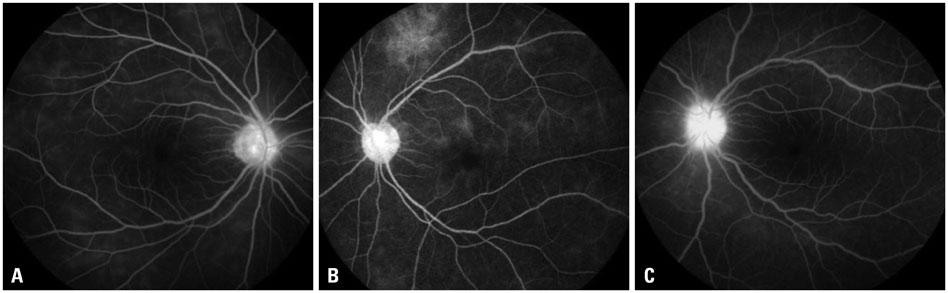
The mean initial VA of eyes with posterior pole-involved vasculitis (63 eyes; 73.3%) was significantly worse than that of those with peripheral vasculitis (23 eye; 26.7%) (logarithm of the minimum angle of resolution VA: 0.554+/-0.572 vs. 0.078+/-0.148; p<0.0001). Subgroup analysis revealed a more severe and diffuse pattern of vascular leakage in posterior pole-involved vasculitis compared to peripheral vasculitis (p<0.0001). Retinal vascular leakage (beta=0.345; p<0.0001), optic disc hyperfluorescence (beta=0.147; p=0.032), and macular leakage (beta=0.107; p=0.047) were significantly associated with worse initial VA. During the follow up (mean: 33.3+/-17.9 months), the change of leakage showed no significant correlation with change of VA in posterior pole-involved vasculitis (tau=0.199, p=0.092).
CONCLUSION
Posterior pole involvement, the degree of retinal vascular leakage, optic disc hyperfluorescence, and macular leakage are significantly associated with VA in Behcet retinal vasculitis.
MeSH Terms
Figure
Reference
-
1. Deuter CM, Kötter I, Wallace GR, Murray PI, Stübiger N, Zierhut M. Behçet's disease: ocular effects and treatment. Prog Retin Eye Res. 2008; 27:111–136.
Article2. Kaçmaz RO, Kempen JH, Newcomb C, Gangaputra S, Daniel E, Levy-Clarke GA, et al. Ocular inflammation in Behçet disease: incidence of ocular complications and of loss of visual acuity. Am J Ophthalmol. 2008; 146:828–836.
Article3. Sakamoto M, Akazawa K, Nishioka Y, Sanui H, Inomata H, Nose Y. Prognostic factors of vision in patients with Behçet disease. Ophthalmology. 1995; 102:317–321.
Article4. Cho YJ, Kim WK, Lee JH, Byeon SH, Koh HJ, Kwon OW, et al. Visual prognosis and risk factors for Korean patients with Behcet uveitis. Ophthalmologica. 2008; 222:344–350.
Article5. Takeuchi M, Hokama H, Tsukahara R, Kezuka T, Goto H, Sakai J, et al. Risk and prognostic factors of poor visual outcome in Behcet's disease with ocular involvement. Graefes Arch Clin Exp Ophthalmol. 2005; 243:1147–1152.
Article6. Zouboulis CC. Adamantiades-Behçet's disease. New York: Kluwer Academic/Plenum Publishers;2003.7. De Laey JJ. Fluorescein angiography in posterior uveitis. Int Ophthalmol Clin. 1995; 35:33–58.
Article8. Demiroğlu H, Barişta I, Dündar S. Risk factor assessment and prognosis of eye involvement in Behcet's disease in Turkey. Ophthalmology. 1997; 104:701–705.
Article9. Atmaca LS, Sonmez PA. Fluorescein and indocyanine green angiography findings in Behçet's disease. Br J Ophthalmol. 2003; 87:1466–1468.
Article10. Bozzoni-Pantaleoni F, Gharbiya M, Pirraglia MP, Accorinti M, Pivetti-Pezzi P. Indocyanine green angiographic findings in Behçet disease. Retina. 2001; 21:230–236.
Article11. Yu HG, Kim MJ, Oh FS. Fluorescein angiography and visual acuity in active uveitis with Behçet disease. Ocul Immunol Inflamm. 2009; 17:41–46.
Article12. International Study Group for Behçet's Disease. Criteria for diagnosis of Behçet's disease. Lancet. 1990; 335:1078–1080.13. Jabs DA, Nussenblatt RB, Rosenbaum JT. Standardization of Uveitis Nomenclature (SUN) Working Group. Standardization of uveitis nomenclature for reporting clinical data Results of the First International Workshop. Am J Ophthalmol. 2005; 140:509–516.
Article14. Suhler EB, Smith JR, Wertheim MS, Lauer AK, Kurz DE, Pickard TD, et al. A prospective trial of infliximab therapy for refractory uveitis: preliminary safety and efficacy outcomes. Arch Ophthalmol. 2005; 123:903–912.
Article15. Tugal-Tutkun I, Herbort CP, Khairallah M. Angiography Scoring for Uveitis Working Group (ASUWOG). Scoring of dual fluorescein and ICG inflammatory angiographic signs for the grading of posterior segment inflammation (dual fluorescein and ICG angiographic scoring system for uveitis). Int Ophthalmol. 2010; 30:539–552.
Article16. Yannuzzi LA. A perspective on the treatment of aphakic cystoid macular edema. Surv Ophthalmol. 1984; 28:Suppl. 540–553.
Article17. Ciardella AP, Prall FR, Borodoker N, Cunningham ET Jr. Imaging techniques for posterior uveitis. Curr Opin Ophthalmol. 2004; 15:519–530.
Article18. Gedik S, Akova Y, Yilmaz G, Bozbeyoğlu S. Indocyanine green and fundus fluorescein angiographic findings in patients with active ocular Behcet's disease. Ocul Immunol Inflamm. 2005; 13:51–58.
Article19. Nussenblatt RB, Kaufman SC, Palestine AG, Davis MD, Ferris FL 3rd. Macular thickening and visual acuity. Measurement in patients with cystoid macular edema. Ophthalmology. 1987; 94:1134–1139.20. Nussenblatt RB, Whitcup SM. Uveitis: fundamentals and clinical practice. 4th ed. Philadelphia: Mosby;2010.21. Spitznas M. Understanding fluorescein angiography. Berlin: Springer;2006.22. Adamus G. Autoantibody-induced apoptosis as a possible mechanism of autoimmune retinopathy. Autoimmun Rev. 2003; 2:63–68.
Article23. Chan CC, Palestine AG, Nussenblatt RB, Roberge FG, Benezra D. Anti-retinal auto-antibodies in Vogt-Koyanagi-Harada syndrome, Behcet's disease, and sympathetic ophthalmia. Ophthalmology. 1985; 92:1025–1028.
Article24. Yilmaz G, Sizmaz S, Yilmaz ED, Duman S, Aydin P. Aqueous humor nitric oxide levels in patients with Behçet disease. Retina. 2002; 22:330–335.
Article25. Rao NA, Wu GS. Free radical mediated photoreceptor damage in uveitis. Prog Retin Eye Res. 2000; 19:41–68.
Article26. Liversidge J, Dick A, Gordon S. Nitric oxide mediates apoptosis through formation of peroxynitrite and Fas/Fas-ligand interactions in experimental autoimmune uveitis. Am J Pathol. 2002; 160:905–916.
Article27. Rajendram R, Saraswathy S, Rao NA. Photoreceptor mitochondrial oxidative stress in early experimental autoimmune uveoretinitis. Br J Ophthalmol. 2007; 91:531–537.
Article28. DiLoreto DA Jr, Bressler NM, Bressler SB, Schachat AP. Use of best and final visual acuity outcomes in ophthalmological research. Arch Ophthalmol. 2003; 121:1586–1590.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravitreal Dexamethasone Implantation in a Behcet's Disease Patient with Macular Edema, Vasculitis after Cataract Surgery
- Frosted Branch Angiitis as Ocular Manifestation of Behcet's Disease: Unusual Case Report and Literature Review
- Multifocal Electrophysiologic Findings in MEWDS: Multiple Evanescent White Dot Syndrome
- Behçet’s Disease Initially Presenting as a Macular Hole with Exudative Retinal Detachment
- Multiple Retinal Arterial Occlusion and Retinal Vasculitis Associated With IgA Nephropathy