Yonsei Med J.
2015 Jul;56(4):913-920. 10.3349/ymj.2015.56.4.913.
Efficacy of Goal-Directed Therapy Using Bioreactance Cardiac Output Monitoring after Valvular Heart Surgery
- Affiliations
-
- 1Department of Thoracic and Cardiovascular Surgery, Cardiovascular Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 2Department of Anesthesiology and Pain Medicine, Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea. aneshim@yuhs.ac
- KMID: 2366329
- DOI: http://doi.org/10.3349/ymj.2015.56.4.913
Abstract
- PURPOSE
We compared the efficacy of postoperative hemodynamic goal-directed therapy (GDT) using a pulmonary artery catheter (PAC) and bioreactance-based noninvasive cardiac output monitoring (NICOM) in patients with atrial fibrillation undergoing valvular heart surgery.
MATERIALS AND METHODS
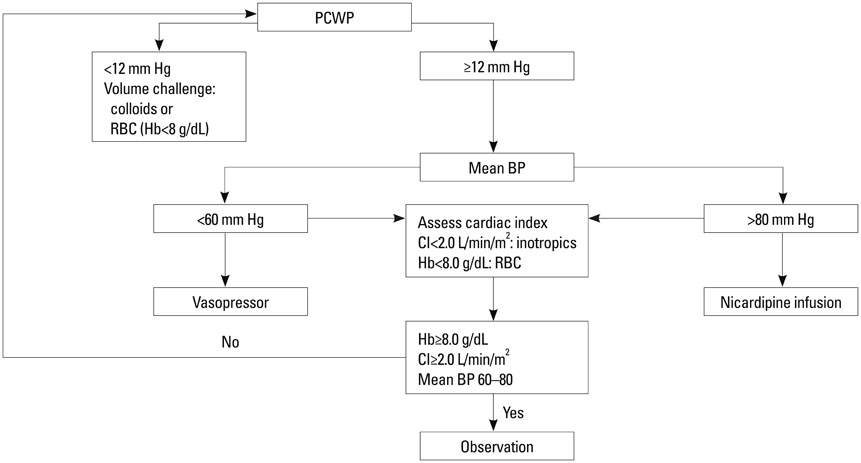
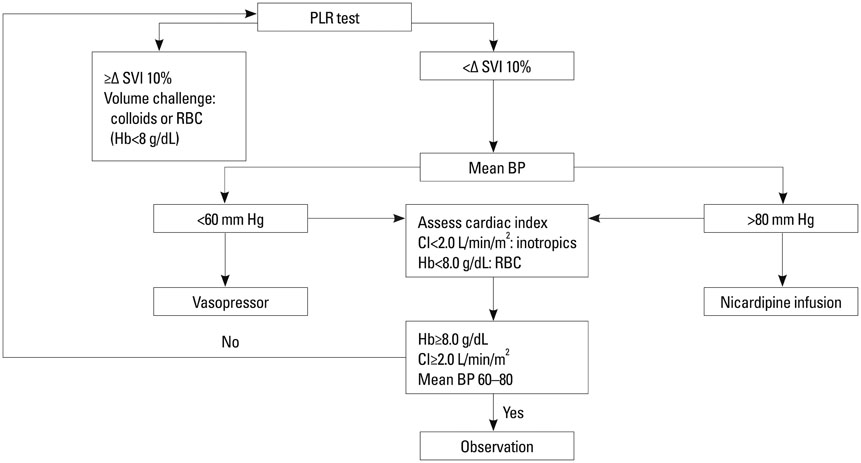
Fifty eight patients were randomized into two groups of GDT with common goals to maintain a mean arterial pressure of 60-80 mm Hg and cardiac index > or =2 L/min/m2: the PAC group (n=29), based on pulmonary capillary wedge pressure, and the NICOM group (n=29), based on changes in stroke volume index after passive leg raising. The primary efficacy variable was length of hospital stay. Secondary efficacy variables included resource utilization including vasopressor and inotropic requirement, fluid balance, and major morbidity endpoints.
RESULTS
Patient characteristics and operative data were similar between the groups, except that significantly more patients underwent double valve replacement in the NICOM group. The lengths of hospital stay were not different between the two groups (12.2+/-4.8 days vs. 10.8+/-4.0 days, p=0.239). Numbers of patients requiring epinephrine (5 vs. 0, p=0.019) and ventilator care >24 h (6 vs. 1, p=0.044) were significantly higher in the PAC group. The PAC group also required significantly larger amounts of colloid (1652+/-519 mL vs. 11430+/-463 mL, p=0.004).
CONCLUSION
NICOM-based postoperative hemodynamic GDT showed promising results in patients with atrial fibrillation undergoing valvular heart surgery in terms of resource utilization.
Keyword
MeSH Terms
-
Aged
Aged, 80 and over
Cardiac Output/*physiology
Cardiac Surgical Procedures/*methods
Catheterization, Swan-Ganz
Female
Goals
Heart Valves/*surgery
Hemodynamics
Humans
Length of Stay/*statistics & numerical data
Male
Middle Aged
Monitoring, Intraoperative/methods
Monitoring, Physiologic/methods
Postoperative Complications/epidemiology/prevention & control
Postoperative Period
Figure
Cited by 1 articles
-
Prosthesis-Patient Mismatch after Mitral Valve Replacement: Comparison of Different Methods of Effective Orifice Area Calculation
In-Jeong Cho, Geu-Ru Hong, Seung Hyun Lee, Sak Lee, Byung-Chul Chang, Chi Young Shim, Hyuk-Jae Chang, Jong-Won Ha, Namsik Chung
Yonsei Med J. 2016;57(2):328-336. doi: 10.3349/ymj.2016.57.2.328.
Reference
-
1. Gan TJ, Soppitt A, Maroof M, el-Moalem H, Robertson KM, Moretti E, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology. 2002; 97:820–826.
Article2. Kapoor PM, Kakani M, Chowdhury U, Choudhury M, Lakshmy , Kiran U. Early goal-directed therapy in moderate to high-risk cardiac surgery patients. Ann Card Anaesth. 2008; 11:27–34.
Article3. Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008; 134:172–178.
Article4. Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011; 39:259–265.
Article5. Warren OJ, Smith AJ, Alexiou C, Rogers PL, Jawad N, Vincent C, et al. The inflammatory response to cardiopulmonary bypass: part 1--mechanisms of pathogenesis. J Cardiothorac Vasc Anesth. 2009; 23:223–231.
Article6. Higgins TL, Yared JP, Ryan T. Immediate postoperative care of cardiac surgical patients. J Cardiothorac Vasc Anesth. 1996; 10:643–658.
Article7. Osman D, Ridel C, Ray P, Monnet X, Anguel N, Richard C, et al. Cardiac filling pressures are not appropriate to predict hemodynamic response to volume challenge. Crit Care Med. 2007; 35:64–68.
Article8. Sandham JD, Hull RD, Brant RF, Knox L, Pineo GF, Doig CJ, et al. A randomized, controlled trial of the use of pulmonary-artery catheters in high-risk surgical patients. N Engl J Med. 2003; 348:5–14.
Article9. Marik PE, Cavallazzi R, Vasu T, Hirani A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: a systematic review of the literature. Crit Care Med. 2009; 37:2642–2647.
Article10. Michard F. Changes in arterial pressure during mechanical ventilation. Anesthesiology. 2005; 103:419–428.
Article11. Kubicek WG, Karnegis JN, Patterson RP, Witsoe DA, Mattson RH. Development and evaluation of an impedance cardiac output system. Aerosp Med. 1966; 37:1208–1212.12. Keren H, Burkhoff D, Squara P. Evaluation of a noninvasive continuous cardiac output monitoring system based on thoracic bioreactance. Am J Physiol Heart Circ Physiol. 2007; 293:H583–H589.
Article13. Raval NY, Squara P, Cleman M, Yalamanchili K, Winklmaier M, Burkhoff D. Multicenter evaluation of noninvasive cardiac output measurement by bioreactance technique. J Clin Monit Comput. 2008; 22:113–119.
Article14. Rich JD, Archer SL, Rich S. Noninvasive cardiac output measurements in patients with pulmonary hypertension. Eur Respir J. 2013; 42:125–133.
Article15. Marik PE, Levitov A, Young A, Andrews L. The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest. 2013; 143:364–370.
Article16. Kim JC, Shim JK, Lee S, Yoo YC, Yang SY, Kwak YL. Effect of combined remote ischemic preconditioning and postconditioning on pulmonary function in valvular heart surgery. Chest. 2012; 142:467–475.
Article17. Warren OJ, Watret AL, de Wit KL, Alexiou C, Vincent C, Darzi AW, et al. The inflammatory response to cardiopulmonary bypass: part 2--anti-inflammatory therapeutic strategies. J Cardiothorac Vasc Anesth. 2009; 23:384–393.
Article18. Levy JH, Tanaka KA. Inflammatory response to cardiopulmonary bypass. Ann Thorac Surg. 2003; 75:S715–S720.
Article19. Montenij LJ, de Waal EE, Buhre WF. Arterial waveform analysis in anesthesia and critical care. Curr Opin Anaesthesiol. 2011; 24:651–656.
Article20. Squara P, Denjean D, Estagnasie P, Brusset A, Dib JC, Dubois C. Noninvasive cardiac output monitoring (NICOM): a clinical validation. Intensive Care Med. 2007; 33:1191–1194.
Article21. Jhanji S, Dawson J, Pearse RM. Cardiac output monitoring: basic science and clinical application. Anaesthesia. 2008; 63:172–181.
Article22. Cavallaro F, Sandroni C, Marano C, La Torre G, Mannocci A, De Waure C, et al. Diagnostic accuracy of passive leg raising for prediction of fluid responsiveness in adults: systematic review and meta-analysis of clinical studies. Intensive Care Med. 2010; 36:1475–1483.
Article23. Monnet X, Rienzo M, Osman D, Anguel N, Richard C, Pinsky MR, et al. Passive leg raising predicts fluid responsiveness in the critically ill. Crit Care Med. 2006; 34:1402–1407.
Article24. Lazor MA, Pierce ET, Stanley GD, Cass JL, Halpern EF, Bode RH Jr. Evaluation of the accuracy and response time of STAT-mode continuous cardiac output. J Cardiothorac Vasc Anesth. 1997; 11:432–436.
Article25. Robin E, Costecalde M, Lebuffe G, Vallet B. Clinical relevance of data from the pulmonary artery catheter. Crit Care. 2006; 10:Suppl 3. S3.26. Di Giantomasso D, Bellomo R, May CN. The haemodynamic and metabolic effects of epinephrine in experimental hyperdynamic septic shock. Intensive Care Med. 2005; 31:454–462.
Article27. Day NP, Phu NH, Bethell DP, Mai NT, Chau TT, Hien TT, et al. The effects of dopamine and adrenaline infusions on acid-base balance and systemic haemodynamics in severe infection. Lancet. 1996; 348:219–223.
Article28. Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013; 41:580–637.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Cardiac Output Measurement
- Recent trend in cardiac output monitoring and trans-cardiopulmonary thermodilution-derived hemodynamic parameters
- Update of Sepsis: Recent Evidences about Early Goal Directed Therapy
- Management of perioperative volume therapy – monitoring and pitfalls
- The Usefulness of Transesophageal Echocardiography During Heart Surgery