Blood Res.
2016 Dec;51(4):261-267. 10.5045/br.2016.51.4.261.
Effect of short-term, high-dose methylprednisolone on oxidative stress in children with acute immune thrombocytopenia
- Affiliations
-
- 1Department of Pediatrics, Harran University Medical Faculty, Sanliurfa, Turkey.
- 2Department of Pediatric Hematology/Oncology, Marmara University Faculty of Medicine, Istanbul, Turkey.
- 3Department of Clinical Biochemistry, Harran University Medical Faculty, Sanliurfa, Turkey.
- 4Department of Pediatric Hematology/Oncology, EskiÅŸehir Osmangazi University Faculty of Medicine, Eskisehir, Turkey. efecanan@yahoo.com
- KMID: 2364317
- DOI: http://doi.org/10.5045/br.2016.51.4.261
Abstract
- BACKGROUND
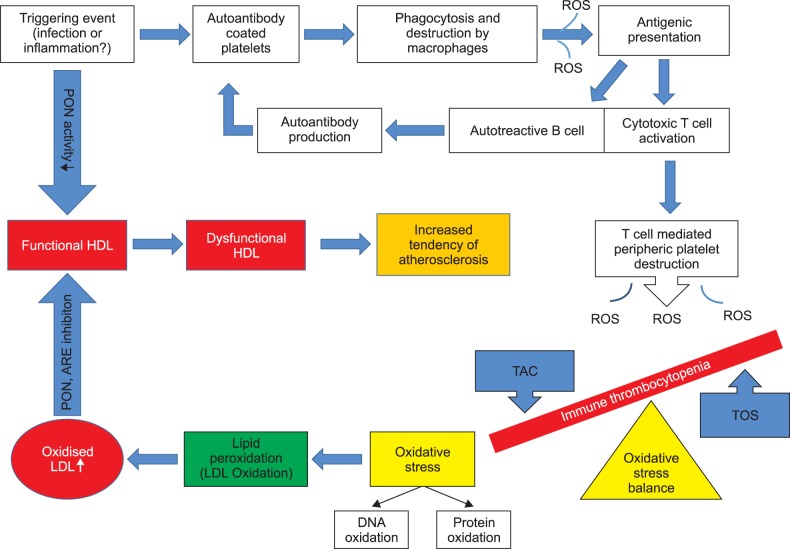
Immune thrombocytopenia (ITP) is the most common cause of acquired childhood thrombocytopenia and is characterized by increased immune-mediated destruction of circulating thrombocytes. Oxidative damage may be involved in ITP pathogenesis; paraoxonase (PON) and arylesterase (ARE) enzymes are closely associated with the cellular antioxidant system. We investigated the effect of short-term high-dose methylprednisolone (HDMP) treatment on the total oxidant status (TOS), total antioxidant capacity (TAC), oxidative stress index (OSI), and PON and ARE enzymatic activity in children with acute ITP.
METHODS
Thirty children with acute ITP constituted the study group and 30 healthy children constituted the control group. Children with acute ITP were treated with HDMP: 30 mg/kg for 3 days, then 20 mg/kg for 4 days. The TOS, TAC, OSI, PON, and ARE levels were determined before and after 7 days of HDMP treatment.
RESULTS
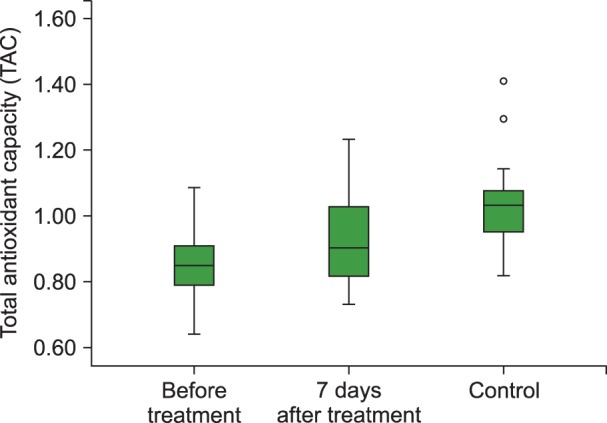
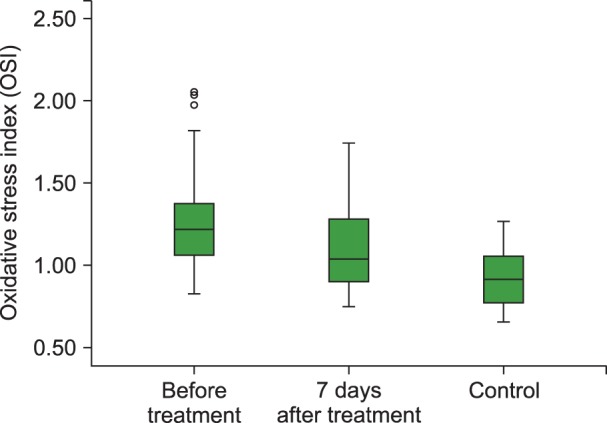
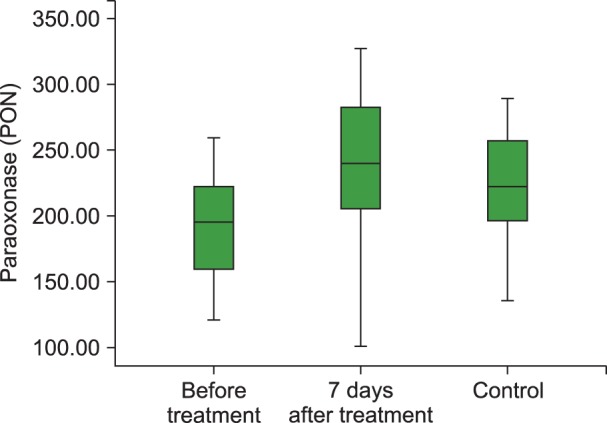
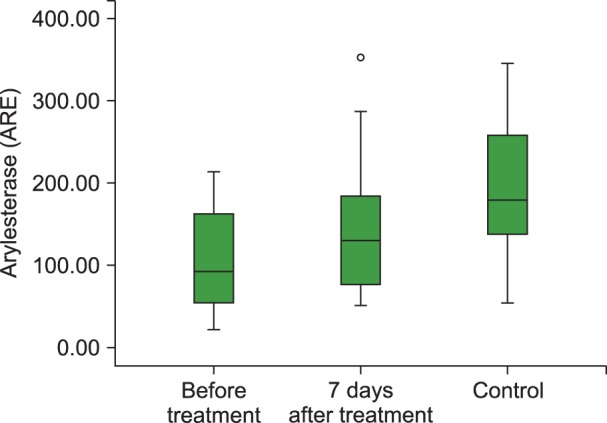
The TAC level (P<0.001), and PON (P<0.001) and ARE (P=0.001) activities were lower and the TOS (P=0.003) and OSI (P<0.001) levels were higher in children with acute ITP than those in healthy children in the control group. We also observed statistically significant increases in the TAC (P<0.01), PON (P<0.001) and ARE levels (P=0.001) and decreases in the TOS (P<0.05) and OSI levels (P<0.05) with 7 days of HDMP treatment compared to their values before treatment.
CONCLUSION
Our study demonstrated increased oxidative stress (OSI and TOC) and decreased antioxidant capacity (TAC), PON, and ARE in ITP patients and that steroid treatment could be effective in reducing the oxidative stress.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Endothelial nitric oxide synthase Glu298Asp gene polymorphism in the cases of idiopathic thrombocytopenic purpura
Saadet Akarsu, Feyzullah Necati Arslan, Deniz Erol
Blood Res. 2022;57(3):223-228. doi: 10.5045/br.2022.2022014.
Reference
-
1. Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009; 113:6511–6521. PMID: 19395674.
Article2. Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: A critical review of published reports. Am J Hematol. 2010; 85:174–180. PMID: 20131303.
Article3. Zhang B, Lo C, Shen L, et al. The role of vanin-1 and oxidative stress-related pathways in distinguishing acute and chronic pediatric ITP. Blood. 2011; 117:4569–4579. PMID: 21325602.
Article4. Kamhieh-Milz J, Bal G, Sterzer V, Kamhieh-Milz S, Arbach O, Salama A. Reduced antioxidant capacities in platelets from patients with autoimmune thrombocytopenia purpura (ITP). Platelets. 2012; 23:184–194. PMID: 21913810.
Article5. Jin CQ, Dong HX, Cheng PP, Zhou JW, Zheng BY, Liu F. Antioxidant status and oxidative stress in patients with chronic ITP. Scand J Immunol. 2013; 77:482–487. PMID: 23551069.
Article6. Akbayram S, Doğan M, Akgün C, et al. The association of oxidant status and antioxidant capacity in children with acute and chronic ITP. J Pediatr Hematol Oncol. 2010; 32:277–281. PMID: 20404751.
Article7. Polat G, Tamer L, Tanriverdi K, Gürkan E, Baslamisli F, Atik U. Levels of malondialdehyde, glutathione and ascorbic acid in idiopathic thrombocytopaenic purpura. East Afr Med J. 2002; 79:446–449. PMID: 12638848.
Article8. Gaidukov L, Tawfik DS. High affinity, stability, and lactonase activity of serum paraoxonase PON1 anchored on HDL with ApoA-I. Biochemistry. 2005; 44:11843–11854. PMID: 16128586.
Article9. Aviram M, Rosenblat M, Billecke S, et al. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free Radic Biol Med. 1999; 26:892–904. PMID: 10232833.
Article10. Lilly SM, Rader DJ. Disorders of HDL metabolism. In : Kwiterovich PO, editor. The Johns Hopkins textbook of dyslipidemia. Philadelphia, PA: Lippincott Williams & Wilkins;2010. p. 105–118.11. Zheng C, Aikawa M. High-density lipoproteins: from function to therapy. J Am Coll Cardiol. 2012; 60:2380–2383. PMID: 23141492.12. Van Lenten BJ, Hama SY, de Beer FC, et al. Anti-inflammatory HDL becomes pro-inflammatory during the acute phase response. Loss of protective effect of HDL against LDL oxidation in aortic wall cell cocultures. J Clin Invest. 1995; 96:2758–2767. PMID: 8675645.
Article13. Mackness B, Davies GK, Turkie W, et al. Paraoxonase status in coronary heart disease: are activity and concentration more important than genotype? Arterioscler Thromb Vasc Biol. 2001; 21:1451–1457. PMID: 11557671.14. Soran H, Younis NN, Charlton-Menys V, Durrington P. Variation in paraoxonase-1 activity and atherosclerosis. Curr Opin Lipidol. 2009; 20:265–274. PMID: 19550323.
Article15. Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004; 37:112–119. PMID: 14725941.
Article16. Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005; 38:1103–1111. PMID: 16214125.
Article17. Eckerson HW, Wyte CM, La Du BN. The human serum paraoxonase/arylesterase polymorphism. Am J Hum Genet. 1983; 35:1126–1138. PMID: 6316781.18. Haagen L, Brock A. A new automated method for phenotyping arylesterase (EC 3.1.1.2) based upon inhibition of enzymatic hydrolysis of 4-nitrophenyl acetate by phenyl acetate. Eur J Clin Chem Clin Biochem. 1992; 30:391–395. PMID: 1525262.
Article19. D'Orazio JA, Neely J, Farhoudi N. ITP in children: pathophysiology and current treatment approaches. J Pediatr Hematol Oncol. 2013; 35:1–13. PMID: 23073045.20. Zhang B, Zehnder JL. Oxidative stress and immune thrombocytopenia. Semin Hematol. 2013; 50:e1–e4. PMID: 23953344.
Article21. Précourt LP, Amre D, Denis MC, et al. The three-gene paraoxonase family: physiologic roles, actions and regulation. Atherosclerosis. 2011; 214:20–36. PMID: 20934178.
Article22. Selek S, Aslan M, Horoz M, Gur M, Erel O. Oxidative status and serum PON1 activity in beta-thalassemia minor. Clin Biochem. 2007; 40:287–291. PMID: 17296173.
Article23. Cakmak A, Soker M, Koc A, Erel O. Paraoxonase and arylesterase activity with oxidative status in children with thalassemia major. J Pediatr Hematol Oncol. 2009; 31:583–587. PMID: 19636263.
Article24. Sarandöl E, Taş S, Dirican M, Serdar Z. Oxidative stress and serum paraoxonase activity in experimental hypothyroidism: effect of vitamin E supplementation. Cell Biochem Funct. 2005; 23:1–8. PMID: 15386442.
Article25. Jarvik GP, Tsai NT, McKinstry LA, et al. Vitamin C and E intake is associated with increased paraoxonase activity. Arterioscler Thromb Vasc Biol. 2002; 22:1329–1333. PMID: 12171796.
Article26. Koc A, Cengiz M, Ozdemir ZC, Celik H. Paraoxonase and arylesterase activities in children with iron deficiency anemia and vitamin B12 deficiency anemia. Pediatr Hematol Oncol. 2012; 29:345–353. PMID: 22568797.27. Alkhouri RH, Baker SS, Hashmi H, Liu W, Baker RD, Zhu L. Paraoxonase gene expression in pediatric inflammatory bowel disease. J Clin Cell Immunol. 2014; 5:224.
Article28. Lim JA, Kim SH. Transcriptional activation of an anti-oxidant mouse Pon2 gene by dexamethasone. BMB Rep. 2009; 42:421–426. PMID: 19643039.
Article29. Sanner BM, Meder U, Zidek W, Tepel M. Effects of glucocorticoids on generation of reactive oxygen species in platelets. Steroids. 2002; 67:715–719. PMID: 12117619.
Article30. Ozsoylu S, Sayli TR, Oztürk G. Oral megadose methylprednisolone versus intravenous immunoglobulin for acute childhood idiopathic thrombocytopenic purpura. Pediatr Hematol Oncol. 1993; 10:317–321. PMID: 8292515.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Short-term, High Dose Methylprednisolone and Narrowband UVB Combination Therapy for 2 Patients with Vitiligo
- Diagnostic Approach of Childhood Immune Thrombocytopenia
- Characteristics of Adverse Effects When Using High Dose Short Term Steroid Regimen
- Acute Effect of Methylprednisolone in Experimental Spinal Cord Injury
- Acute Effect of Methylprednisolone in Experimental Spinal Cord Injury