J Vet Sci.
2016 Mar;17(1):89-96. 10.4142/jvs.2016.17.1.89.
Production of α1,3-galactosyltransferase targeted pigs using transcription activator-like effector nuclease-mediated genome editing technology
- Affiliations
-
- 1MGENPLUS Biotechnology Research Institute, Seoul 08511, Korea. parkkw@scnu.kr
- 2Department of Animal Science & Technology, Sunchon National University, Suncheon 57922, Korea.
- 3Department of Animal and Poultry Sciences, Virginia Tech, Blacksburg, VA 24061, USA.
- KMID: 2363339
- DOI: http://doi.org/10.4142/jvs.2016.17.1.89
Abstract
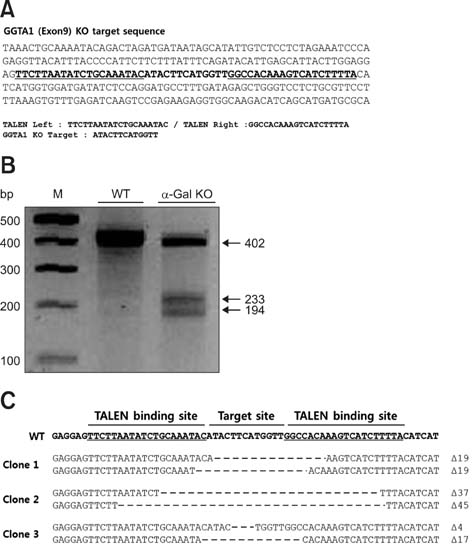
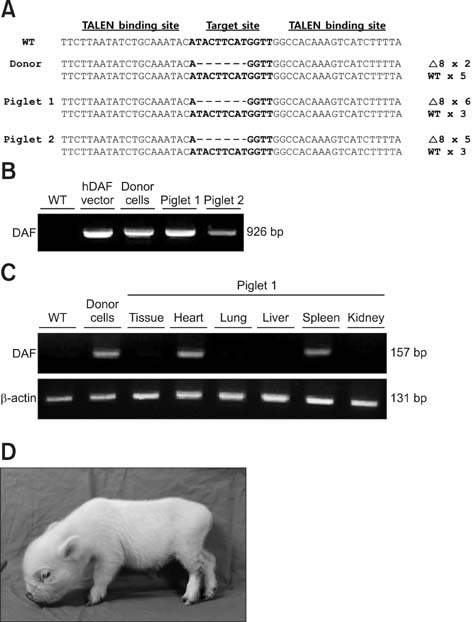
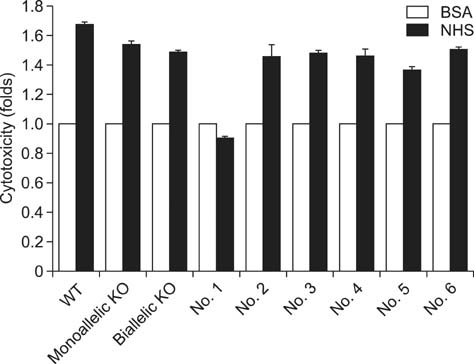
- Recent developments in genome editing technology using meganucleases demonstrate an efficient method of producing gene edited pigs. In this study, we examined the effectiveness of the transcription activator-like effector nuclease (TALEN) system in generating specific mutations on the pig genome. Specific TALEN was designed to induce a double-strand break on exon 9 of the porcine α1,3-galactosyltransferase (GGTA1) gene as it is the main cause of hyperacute rejection after xenotransplantation. Human decay-accelerating factor (hDAF) gene, which can produce a complement inhibitor to protect cells from complement attack after xenotransplantation, was also integrated into the genome simultaneously. Plasmids coding for the TALEN pair and hDAF gene were transfected into porcine cells by electroporation to disrupt the porcine GGTA1 gene and express hDAF. The transfected cells were then sorted using a biotin-labeled IB4 lectin attached to magnetic beads to obtain GGTA1 deficient cells. As a result, we established GGTA1 knockout (KO) cell lines with biallelic modification (35.0%) and GGTA1 KO cell lines expressing hDAF (13.0%). When these cells were used for somatic cell nuclear transfer, we successfully obtained live GGTA1 KO pigs expressing hDAF. Our results demonstrate that TALEN-mediated genome editing is efficient and can be successfully used to generate gene edited pigs.
Keyword
MeSH Terms
-
Animals
Antigens, CD55/genetics
Cell Line
DNA Breaks, Double-Stranded
Exons/genetics
Galactosyltransferases/*genetics
Gene Editing/*veterinary
Gene Knockout Techniques
Humans
Nuclear Transfer Techniques
Swine
Transcription Activator-Like Effector Nucleases/*genetics/*metabolism
Antigens, CD55
Galactosyltransferases
Transcription Activator-Like Effector Nucleases
Figure
Cited by 1 articles
-
Improved preimplantation development of porcine somatic cell nuclear transfer embryos by caffeine treatment
Ghangyong Kim, Pantu Kumar Roy, Xun Fang, Bahia MS Hassan, Jongki Cho
J Vet Sci. 2019;20(3):. doi: 10.4142/jvs.2019.20.e31.
Reference
-
1. Aigner B, Klymiuk N, Wolf E. Transgenic pigs for xenotransplantation: selection of promoter sequences for reliable transgene expression. Curr Opin Organ Transplant. 2010; 15:201–206.
Article2. Baldan N, Rigotti P, Calabrese F, Cadrobbi R, Dedja A, Iacopetti I, Boldrin M, Seveso M, Dall'Olmo L, Frison L, De Benedictis G, Bernardini D, Thiene G, Cozzi E, Ancona E. Ureteral stenosis in HDAF pig-to-primate renal xenotransplantation: a phenomenon related to immunological events? Am J Transplant. 2004; 4:475–481.
Article3. Boch J, Bonas U. Xanthomonas AvrBs3 family-type III effectors: discovery and function. Annu Rev Phytopathol. 2010; 48:419–436.4. Carlson DF, Tan W, Lillico SG, Stverakova D, Proudfoot C, Christian M, Voytas DF, Long CR, Whitelaw CB, Fahrenkrug SC. Efficient TALEN-mediated gene knockout in livestock. Proc Natl Acad Sci U S A. 2012; 109:17382–17387.
Article5. Chen G, Sun H, Yang H, Kubelik D, Garcia B, Luo Y, Xiang Y, Qian A, Copeman L, Liu W, Cardella CJ, Wang W, Xiong Y, Wall W, White DJ, Zhong R. The role of anti-non-Gal antibodies in the development of acute humoral xenograft rejection of hDAF transgenic porcine kidneys in baboons receiving anti-Gal antibody neutralization therapy. Transplantation. 2006; 81:273–283.
Article6. Galili U. Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today. 1993; 14:480–482.
Article7. Galili U, Shohet SB, Kobrin E, Stults CLM, Macher BA. Man, apes, and Old World monkeys differ from other mammals in the expression of α-galactosyl epitopes on nucleated cells. J Biol Chem. 1988; 263:17755–17762.
Article8. Geurts AM, Cost GJ, Freyvert Y, Zeitler B, Miller JC, Choi VM, Jenkins SS, Wood A, Cui X, Meng X, Vincent A, Lam S, Michalkiewicz M, Schilling R, Foeckler J, Kalloway S, Weiler H, Ménoret S, Anegon I, Davis GD, Zhang L, Rebar EJ, Gregory PD, Urnov FD, Jacob HJ, Buelow R. Knockout rats via embryo microinjection of zinc-finger nucleases. Science. 2009; 325:433.
Article9. Gouble A, Smith J, Bruneau S, Perez C, Guyot V, Cabaniols JP, Leduc S, Fiette L, Avé P, Micheau B, Duchateau P, Pâques F. Efficient in toto targeted recombination in mouse liver by meganuclease-induced double-strand break. J Gene Med. 2006; 8:616–622.
Article10. Hauschild J, Petersen B, Santiago Y, Queisser AL, Carnwath JW, Lucas-Hahn A, Zhang L, Meng X, Gregory PD, Schwinzer R, Cost GJ, Niemann H. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proc Natl Acad Sci U S A. 2011; 108:12013–12017.
Article11. Huang P, Xiao A, Zhou M, Zhu Z, Lin S, Zhang B. Heritable gene targeting in zebrafish using customized TALENs. Nat Biotechnol. 2011; 29:699–700.
Article12. Kolber-Simonds D, Lai L, Watt SR, Denaro M, Arn S, Augenstein ML, Betthauser J, Carter DB, Greenstein JL, Hao Y, Im GS, Liu Z, Mell GD, Murphy CN, Park KW, Rieke A, Ryan DJJ, Sachs DH, Forsberg EJ, Prather RS, Hawley RJ. Production of α-1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc Natl Acad Sci U S A. 2004; 101:7335–7340.
Article13. Kuwaki K, Tseng YL, Dor FJMF, Shimizu A, Houser SL, Sanderson TM, Lancos CJ, Prabharasuth DD, Cheng J, Moran K, Hisashi Y, Mueller N, Yamada K, Greenstein JL, Hawley RJ, Patience C, Awwad M, Fishman JA, Robson SC, Schuurman HJ, Sachs DH, Cooper DKC. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005; 11:29–31.
Article14. Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, Samuel M, Bonk A, Rieke A, Day BN, Murphy CN, Carter DB, Hawley RJ, Prather RS. Production of α-1,3-galactosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002; 295:1089–1092.
Article15. Lavitrano M, Bacci ML, Forni M, Lazzereschi D, Di Stefano C, Fioretti D, Giancotti P, Marfé G, Pucci L, Renzi L, Wang H, Stoppacciaro A, Stassi G, Sargiacomo M, Sinibaldi P, Turchi V, Giovannoni R, Della Casa G, Seren E, Rossi G. Efficient production by sperm-mediated gene transfer of human decay accelerating factor (hDAF) transgenic pigs for xenotransplantation. Proc Natl Acad Sci U S A. 2002; 99:14230–14235.
Article16. Le Bas-Bernardet S, Anegon I, Blancho G. Progress and prospects: genetic engineering in xenotransplantation. Gene Ther. 2008; 15:1247–1256.
Article17. Lee K, Kwon DN, Ezashi T, Choi YJ, Park C, Ericsson AC, Brown AN, Samuel MS, Park KW, Walters EM, Kim DY, Kim JH, Franklin CL, Murphy CN, Roberts RM, Prather RS, Kim JH. Engraftment of human iPS cells and allogeneic porcine cells into pigs with inactivated RAG2 and accompanying severe combined immunodeficiency. Proc Natl Acad Sci U S A. 2014; 111:7260–7265.
Article18. Lei Y, Guo X, Liu Y, Cao Y, Deng Y, Chen X, Cheng CHK, Dawid IB, Chen Y, Zhao H. Efficient targeted gene disruption in Xenopus embryos using engineered transcription activator-like effector nucleases (TALENs). Proc Natl Acad Sci U S A. 2012; 109:17484–17489.
Article19. Lillico SG, Proudfoot C, Carlson DF, Stverakova D, Neil C, Blain C, King TJ, Ritchie WA, Tan W, Mileham AJ, McLaren DG, Fahrenkrug SC, Whitelaw CBA. Live pigs produced from genome edited zygotes. Sci Rep. 2013; 3:2847.
Article20. Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, Sharma SB, Lamborn AE, Garst AS, Moore M, Demetris AJ, Rudert WA, Bottino R, Bertera S, Trucco M, Starzl TE, Dai Y, Ayares DL. Production of α1,3-galactosyltransferase-deficient pigs. Science. 2003; 299:411–414.
Article21. Reyes LM, Estrada JL, Ivary B, Sidner RA, Paris LL, Tector AJ. Efficient selection of Gal-knockout pig cells for somatic cell nuclear transfer. J Surg Res. 2013; 184:e37–e42.
Article22. Song J, Zhong J, Guo X, Chen Y, Zou Q, Huang J, Li X, Zhang Q, Jiang Z, Tang C, Yang H, Liu T, Li P, Pei D, Lai L. Generation of RAG 1- and 2-deficient rabbits by embryo microinjection of TALENs. Cell Res. 2013; 23:1059–1062.
Article23. Sprangers B, Waer M, Billiau AD. Xenotransplantation: where are we in 2008? Kidney Int. 2008; 74:14–21.
Article24. Sung YH, Baek IJ, Kim DH, Jeon J, Lee J, Lee K, Jeong D, Kim JS, Lee HW. Knockout mice created by TALEN-mediated gene targeting. Nat Biotechnol. 2013; 31:23–24.
Article25. Tan W, Carlson DF, Lancto CA, Garbe JR, Webster DA, Hackett PB, Fahrenkrug SC. Efficient nonmeiotic allele introgression in livestock using custom endonucleases. Proc Natl Acad Sci U S A. 2013; 110:16526–16531.
Article26. Tesson L, Usal C, Ménoret S, Leung E, Niles BJ, Remy S, Santiago Y, Vincent AI, Meng X, Zhang L, Gregory PD, Anegon I, Cost GJ. Knockout rats generated by embryo microinjection of TALENs. Nat Biotechnol. 2011; 29:695–696.
Article27. Whitworth KM, Lee K, Benne JA, Beaton BP, Spate LD, Murphy SL, Samuel MS, Mao J, O'Gorman C, Walters EM, Murphy CN, Driver J, Mileham A, McLaren D, Wells KD, Prather RS. Use of the CRISPR/Cas9 system to produce genetically engineered pigs from in vitro-derived oocytes and embryos. Biol Reprod. 2014; 91:78.28. Xin J, Yang H, Fan N, Zhao B, Ouyang Z, Liu Z, Zhao Y, Li X, Song J, Yang Y, Zou Q, Yan Q, Zeng Y, Lai L. Highly efficient generation of GGTA1 biallelic knockout inbred mini-pigs with TALENs. PLoS One. 2013; 8:e84250.29. Yang YG, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007; 7:519–531.
Article30. Yao J, Huang J, Hai T, Wang X, Qin G, Zhang H, Wu R, Cao C, Xi JJ, Yuan Z, Zhao J. Efficient bi-allelic gene knockout and site-specific knock-in mediated by TALENs in pigs. Sci Rep. 2014; 4:6926.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Genome Editing Using Engineered Nucleases
- Generation of knockout mouse models of cyclin-dependent kinase inhibitors by engineered nuclease-mediated genome editing
- Genome editing: the road of CRISPR/Cas9 from bench to clinic
- Applications of CRISPR/Cas9 for Gene Editing in Hereditary Movement Disorders
- Electroporation of AsCpf1/RNP at the Zygote Stage is an Efficient Genome Editing Method to Generate Knock-Out Mice Deficient in Leukemia Inhibitory Factor