J Vet Sci.
2016 Mar;17(1):21-26. 10.4142/jvs.2016.17.1.21.
Improved immunogenicity of Newcastle disease virus inactivated vaccine following DNA vaccination using Newcastle disease virus hemagglutinin-neuraminidase and fusion protein genes
- Affiliations
-
- 1Department of Pathobiology, Faculty of Veterinary Medicine, University of Tabriz, Tabriz 5166616471, Iran.
- 2Institute of Bioscience, Universiti Putra Malaysia, 43400 Serdang, Malaysia. aiini@upm.my
- 3Razi Vaccine and Serum Research Institute, Arak 3197619751, Iran.
- 4Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Putra Malaysia, 43400 Serdang, Malaysia.
- KMID: 2363331
- DOI: http://doi.org/10.4142/jvs.2016.17.1.21
Abstract
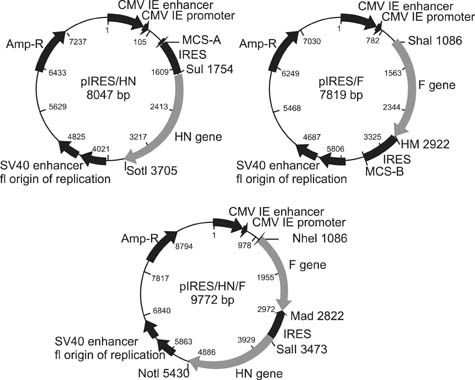
- The present study describes the development of DNA vaccines using the hemagglutinin-neuraminidase (HN) and fusion (F) genes from AF2240 Newcastle disease virus strain, namely pIRES/HN, pIRES/F and pIRES-F/HN. Transient expression analysis of the constructs in Vero cells revealed the successful expression of gene inserts in vitro. Moreover, in vivo experiments showed that single vaccination with the constructed plasmid DNA (pDNA) followed by a boost with inactivated vaccine induced a significant difference in enzyme-linked immunosorbent assay antibody levels (p < 0.05) elicited by either pIRES/F, pIRES/F+ pIRES/HN or pIRES-F/HN at one week after the booster in specific pathogen free chickens when compared with the inactivated vaccine alone. Taken together, these results indicated that recombinant pDNA could be used to increase the efficacy of the inactivated vaccine immunization procedure.
MeSH Terms
-
Animals
Antibodies, Viral/blood
Cercopithecus aethiops
Chickens
*HN Protein/genetics/immunology
Immunogenicity, Vaccine/*immunology
Newcastle Disease/immunology
Newcastle disease virus/enzymology/*genetics/immunology
Specific Pathogen-Free Organisms
Vaccines, DNA/genetics/*immunology
Vaccines, Inactivated/immunology
Vero Cells
*Viral Fusion Proteins/genetics/immunology
Viral Vaccines/genetics/*immunology/*standards
Antibodies, Viral
HN Protein
Vaccines, DNA
Vaccines, Inactivated
Viral Fusion Proteins
Viral Vaccines
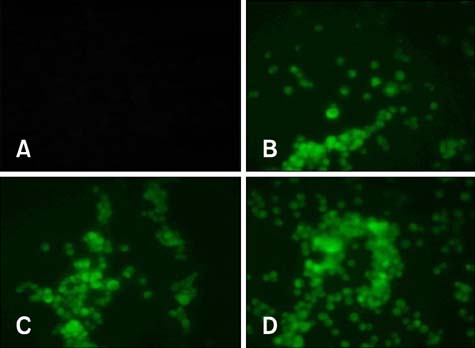
Figure
Reference
-
1. Alexander D. Newcastle disease and other avian Paramyxoviridae infection. In : Calnek BW, Barnes HJ, Beard CW, McDougald LR, Saif YM, editors. Diseases of Poultry. 10th ed. Ames: Iowa State University Press;1997. p. 541–549.2. Arora P, Lakhchaura BD, Garg SK. Evaluaion of immunogenic potential of 75kDa and 56kDa proteins of Newcastle disease virus (NDV). Indian J Exp Biol. 2010; 48:889–895.3. Boursnell ME, Green PF, Samson AC, Campbell JI, Deuter A, Peters RW, Millar NS, Emmerson PT, Binns MM. A recombinant fowlpox virus expressing the hemagglutinin-neuraminidase gene of Newcastle disease virus (NDV) protects chickens against challenge by NDV. Virology. 1990; 178:297–300.
Article4. Cosset FL, Bouquet JF, Drynda A, Chebloune Y, Rey-Senelonge A, Kohen G, Nigon VM, Desmettre P, Verdier G. Newcastle disease virus (NDV) vaccine based on immunization with avian cells expressing the NDV hemagglutinin-neuraminidase glycoprotein. Virology. 1991; 185:862–866.
Article5. Donnelly JJ, Wahren B, Liu MA. DNA vaccines: progress and challenges. J Immunol. 2005; 175:633–639.
Article6. Dortmans JCFM, Koch G, Rottier PJM, Peeters BPH. Virulence of Newcastle disease virus: what is known so far? Vet Res. 2011; 42:122.
Article7. Gurunathan S, Klinman DM, Seder RA. DNA vaccines: immunology, application, and optimization. Annu Rev Immunol. 2000; 18:927–974.
Article8. Jazayeri SD, Ideris A, Zakaria Z, Shameli K, Moeini H, Omar AR. Cytotoxicity and immunological responses following oral vaccination of nanoencapsulated avian influenza virus H5 DNA vaccine with green synthesis silver nanoparticles. J Control Release. 2012; 161:116–123.
Article9. Krishnamurthy S, Huang Z, Samal SK. Recovery of a virulent strain of Newcastle disease virus from cloned cDNA: expression of a foreign gene results in growth retardation and attenuation. Virology. 2000; 278:168–182.
Article10. Loke CF, Omar AR, Raha AR, Yusoff K. Improved protection from velogenic Newcastle disease virus challenge following multiple immunizations with plasmid DNA encoding for F and HN genes. Vet Immunol Immunopathol. 2005; 106:259–267.
Article11. Moeini H, Omar AR, Rahim RA, Yusoff K. Development of a DNA vaccine against chicken anemia virus by using a bicistronic vector expressing VP1 and VP2 proteins of CAV. Comp Immunol Microbiol Infect Dis. 2011; 34:227–236.
Article12. Moeini H, Omar AR, Rahim RA, Yusoff K. Improving the potency of DNA vaccine against chicken anemia virus (CAV) by fusing VP1 protein of CAV to Marek's disease virus (MDV) type-1 VP22 protein. Virol J. 2011; 8:119.
Article13. Morgan RW, Gelb J Jr, Schreurs CS, Lütticken D, Rosenberger JK, Sondermeijer PJ. Protection of chickens from Newcastle and Marek's diseases with a recombinant herpesvirus of turkeys vaccine expressing the Newcastle disease virus fusion protein. Avian Dis. 1992; 36:858–870.
Article14. Nagai Y, Klenk HD, Rott R. Proteolytic cleavage of the viral glycoproteins and its significance for the virulence of Newcastle disease virus. Virology. 1976; 72:494–508.
Article15. Nakaya T, Cros J, Park MS, Nakaya Y, Zheng H, Sagrera A, Villar E, García-Sastre A, Palese P. Recombinant Newcastle disease virus as a vaccine vector. J Virol. 2001; 75:11868–11873.
Article16. Pedersen N, Hansen S, Heydenreich AV, Kristensen HG, Poulsen HS. Solid lipid nanoparticles can effectively bind DNA, streptavidin and biotinylated ligands. Eur J Pharm Biopharm. 2006; 62:155–162.
Article17. Rajawat YS, Sundaresan NR, Ravindra PV, Kantaraja C, Ratta B, Sudhagar M, Rai A, Saxena VK, Palia SK, Tiwari AK. Immune responses induced by DNA vaccines encoding Newcastle virus haemagglutinin and/or fusion proteins in maternal antibody-positive commercial broiler chicken. Br Poult Sci. 2008; 49:111–117.
Article18. Reddy GS, Srinivasan VA. Use of BHK cell culture-adapted Newcastle disease virus for immunization of chicks. Vaccine. 1992; 10:164–166.
Article19. Rees S, Coote J, Stables J, Goodson S, Harris S, Lee MG. Bicistronic vector for the creation of stable mammalian cell lines that predisposes all antibiotic-resistant cells to express recombinant protein. Biotechniques. 1996; 20:102–104. 106108110
Article20. Reynolds DL, Maraqa AD. Protective immunity against Newcastle disease: the role of antibodies specific to Newcastle disease virus polypeptides. Avian Dis. 2000; 44:138–144.
Article21. Reynolds DL, Maraqa AD. Protective immunity against Newcastle disease: the role of cell-mediated immunity. Avian Dis. 2000; 44:145–154.
Article22. Richter E, Wick G. Fluoro-immuno-cytoadherence (FICA): a new method for the identification and enumeration of antigen-binding cells. Z Immunitatsforsch Immunobiol. 1977; 152:351–362.
Article23. Sakaguchi M, Nakamura H, Sonoda K, Hamada F, Hirai K. Protection of chickens from Newcastle disease by vaccination with a linear plasmid DNA expressing the F protein of Newcastle disease virus. Vaccine. 1996; 14:747–752.
Article24. Sawant PM, Verma PC, Subudhi PK, Chaturvedi U, Singh M, Kumar R, Tiwari AK. Immunomodulation of bivalent Newcastle disease DNA vaccine induced immune response by co-delivery of chicken IFN-γ and IL-4 genes. Vet Immunol Immunopathol. 2011; 144:36–44.
Article25. Stone-Hulslander J, Morrison TG. Detection of an interaction between the HN and F proteins in Newcastle disease virus-infected cells. J Virol. 1997; 71:6287–6295.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterisation of genotype VII Newcastle disease virus (NDV) isolated from NDV vaccinated chickens, and the efficacy of LaSota and recombinant genotype VII vaccines against challenge with velogenic NDV
- Cloning and Expression of Hemagglutinin-Neuraminidase Gene of a Thermostable Isolate of Newcastle Disease Virus by Baculovirus Recombinants
- Newcastle disease virus vectored vaccines as bivalent or antigen delivery vaccines
- Transient expression of fusion and hemagglutinin-neuraminidase epitopes of Newcastle disease virus in maize as a potent candidate vaccine
- Expression of F Protein Gene of a Thermostable Isolate of Newcastle Disease Virus Using Baculovirus Expression System