Clin Exp Otorhinolaryngol.
2015 Jun;8(2):129-135. 10.3342/ceo.2015.8.2.129.
A Comparison of Cefditoren Pivoxil 8-12 mg/kg/day and Cefditoren Pivoxil 16-20 mg/kg/day in Treatment of Children With Acute Presumed Bacterial Rhinosinusitis: A Prospective, Randomized, Investigator-Blinded, Parallel-Group Study
- Affiliations
-
- 1Division of Allergy and Immunology, Department of Pediatrics, Faculty of Medicine, Thammasat University, Klong Luang, Thailand. orapanpoachanukoon@yahoo.com
- 2Division of Infectious Disease, Department of Pediatrics, Faculty of Medicine, Thammasat University, Klong Luang, Thailand.
- 3Research Office, Faculty of Medicine, Thammasat University, Klong Luang, Thailand.
- KMID: 2360783
- DOI: http://doi.org/10.3342/ceo.2015.8.2.129
Abstract
OBJECTIVES
Cefditoren pivoxil (CDT) has been used in the treatment of rhinosinusitis. However, little is known about the efficacy of this drug at low and high doses. This study was to compare the efficacy and safety of low dose (8-12 mg/kg/day) and high dose (16-20 mg/kg/day) CDT in the treatment of children with uncomplicated acute rhinosinusitis (ARS).
METHODS
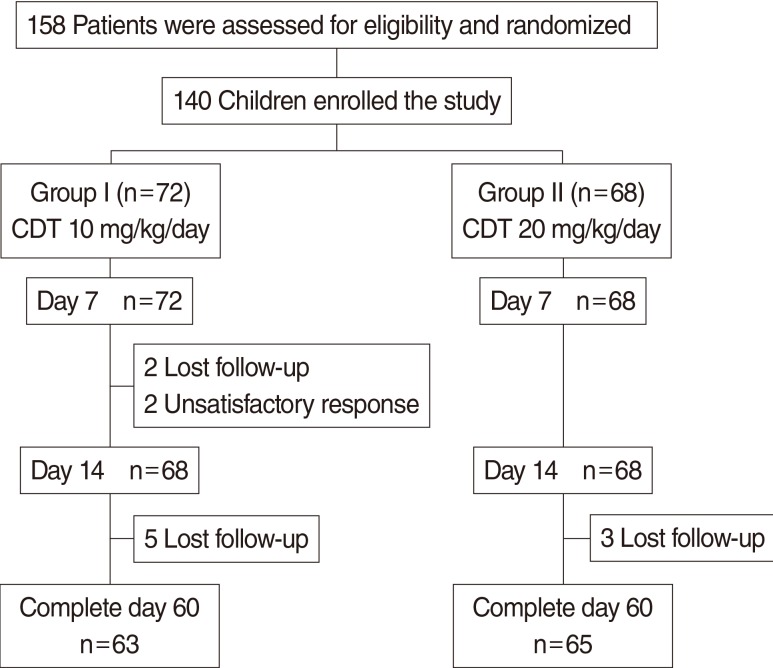
This investigation was a randomized, investigator-blinded, and parallel study, conducted in patients (aged 1-15 years) with a clinical diagnosis of uncomplicated ARS. Two groups of patients randomly received low dose or high dose CDT for 14 days. Patients' symptoms were assessed quantitatively using a quantitative symptom score (the S5 score). The changes in sinus symptoms and adverse events were provided by patients and their parents/caregivers. The response rate and adverse effects were evaluated at days 7 and 14. The relapse rate was recorded at days 21 and 28. The recurrences of sinus symptoms at day 60 were also assessed.
RESULTS
One hundred forty patients were recruited and randomized; 72 received low dose CDT (group I) and 68 received high dose CDT (group II). There were no significant differences in demographic data including sex, age, presenting symptoms, medical history, and X-ray findings between two groups. The responses rate at day 14 in groups I and II were 95.5% and 95.4%, respectively (P>0.99). There were no significant differences between groups in relapse rate at day 28 and no recurrence at day 60 in either group. The most common treatment-related adverse events were diarrhea (4.2% in group I vs. 2.9% in group II) and vomiting (2.8% in group I vs. 10.3% in group II). There was no statistically significant difference in adverse events between groups.
CONCLUSION
Both low and high doses regimens of CDT appeared a similar clinical outcome for treatment in uncomplicated ARS in pediatric patients.
Keyword
MeSH Terms
Figure
Reference
-
1. Jean SS, Hsueh PR. High burden of antimicrobial resistance in Asia. Int J Antimicrob Agents. 2011; 4. 37(4):291–295. PMID: 21382699.
Article2. Felmingham D, Gruneberg RN. The Alexander Project 1996-1997: latest susceptibility data from this international study of bacterial pathogens from community-acquired lower respiratory tract infections. J Antimicrob Chemother. 2000; 2. 45(2):191–203. PMID: 10660501.
Article3. Felmingham D. Evolving resistance patterns in community-acquired respiratory tract pathogens: first results from the PROTEKT global surveillance study. Prospective Resistant Organism Tracking and Epidemiology for the Ketolide Telithromycin. J Infect. 2002; 2. 44(Suppl A):3–10. PMID: 12150493.4. Wang H, Chen M, Xu Y, Sun H, Yang Q, Hu Y, et al. Antimicrobial susceptibility of bacterial pathogens associated with community-acquired respiratory tract infections in Asia: report from the Community-Acquired Respiratory Tract Infection Pathogen Surveillance (CARTIPS) study, 2009-2010. Int J Antimicrob Agents. 2011; 11. 38(5):376–383. PMID: 21880469.
Article5. Dejsirilert S, Tienkrim S, Ubonyaem N, Sawanpanyalert P, Aswapokee N, Suankratay C. National antimicrobial resistance surveillance among clinical isolates of Streptococcus pneumoniae in Thailand. J Med Assoc Thai. 2009; 8. 92(Suppl 4):S19–S33. PMID: 21298844.6. Chow AW, Benninger MS, Brook I, Brozek JL, Goldstein EJ, Hicks LA, et al. IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012; 4. 54(8):e72–e112. PMID: 22438350.
Article7. Wellington K, Curran MP. Cefditoren pivoxil: a review of its use in the treatment of bacterial infections. Drugs. 2004; 64(22):2597–2618. PMID: 15516158.8. Soriano F, Granizo JJ, Fenoll A, Gracia M, Fernandez-Roblas R, Esteban J, et al. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae isolated in four southern European countries (ARISE project) from adult patients: results from the cefditoren surveillance program. J Chemother. 2003; 4. 15(2):107–112. PMID: 12797384.9. Seral C, Suarez L, Rubio-Calvo C, Gomez-Lus R, Gimeno M, Coronel P, et al. In vitro activity of cefditoren and other antimicrobial agents against 288 Streptococcus pneumoniae and 220 Haemophilus influenzae clinical strains isolated in Zaragoza, Spain. Diagn Microbiol Infect Dis. 2008; 10. 62(2):210–215. PMID: 18715733.
Article10. Gracia M, Diaz C, Coronel P, Gimeno M, Garcia-Rodas R, del Prado G, et al. Antimicrobial susceptibility of Haemophilus influenzae and Moraxella catarrhalis isolates in eight Central, East and Baltic European countries in 2005-06: results of the Cefditoren Surveillance Study. J Antimicrob Chemother. 2008; 5. 61(5):1180–1181. PMID: 18316820.
Article11. Kang JH, Lee SY, Kim JH, Hur JK, Lee KY. In vitro antimicrobial activity of cefditoren and other oral antibiotics against Streptococcus pneumoniae, isolated from children with community acquired respiratory tract infections. Jpn J Antibiot. 2010; 2. 63(1):11–17. PMID: 20836403.12. Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998; 1. 26(1):1–10. PMID: 9455502.13. Heffelfinger JD, Dowell SF, Jorgensen JH, Klugman KP, Mabry LR, Musher DM, et al. Management of community-acquired pneumonia in the era of pneumococcal resistance: a report from the Drug-Resistant Streptococcus pneumoniae Therapeutic Working Group. Arch Intern Med. 2000; 5. 160(10):1399–1408. PMID: 10826451.14. Fujii R, Yoshioka H, Okuno A, Fujita K, Murono K, Maruyama S, et al. Pharmacokinetic and clinical studies of cefditoren pivoxil in the pediatric field: Pediatric Study Group of ME1207. Jpn J Antibiot. 1993; 1. 46(1):95–114. PMID: 8455336.15. Soriano F, Gimenez MJ, Aguilar L. Cefditoren in upper and lower community-acquired respiratory tract infections. Drug Des Devel Ther. 2011; 2. 5:85–94.
Article16. Sadaba B, Azanza JR, Quetglas EG, Campanero MA, Honorato J, Coronel P, et al. Pharmacokinetic/pharmacodynamic serum and urine profile of cefditoren following single-dose and multiple twice- and thrice-daily regimens in healthy volunteers: a phase I study. Rev Esp Quimioter. 2007; 3. 20(1):51–60. PMID: 17530036.17. Garbutt JM, Gellman EF, Littenberg B. The development and validation of an instrument to assess acute sinus disease in children. Qual Life Res. 1999; 5. 8(3):225–233. PMID: 10472153.18. Wongsawat J, Chokephaibulkit K. Implication of pneumococcal conjugate vaccines to public health: Thailand perspective. J Med Assoc Thai. 2010; 11. 93(Suppl 5):S53–S60. PMID: 21294383.19. Fenoll A, Aguilar L, Robledo O, Gimenez MJ, Tarrago D, Granizo JJ, et al. Influence of the beta-lactam resistance phenotype on the cefuroxime versus cefditoren susceptibility of Streptococcus pneumoniae and Haemophilus influenzae recovered from children with acute otitis media. J Antimicrob Chemother. 2007; 8. 60(2):323–327. PMID: 17562681.20. Schrag SJ, Pena C, Fernandez J, Sanchez J, Gomez V, Perez E, et al. Effect of short-course, high-dose amoxicillin therapy on resistant pneumococcal carriage: a randomized trial. JAMA. 2001; 7. 286(1):49–56. PMID: 11434826.21. Poachanukoon O, Kitcharoensakkul M. Efficacy of cefditoren pivoxil and amoxicillin/clavulanate in the treatment of pediatric patients with acute bacterial rhinosinusitis in Thailand: a randomized, investigator-blinded, controlled trial. Clin Ther. 2008; 10. 30(10):1870–1879. PMID: 19014842.
Article22. Felmingham D, Robbins MJ, Ghosh G, Bhogal H, Mehta MD, Leakey A, et al. An in vitro characterization of cefditoren, a new oral cephalosporin. Drugs Exp Clin Res. 1994; 20(4):127–147. PMID: 7813385.23. Bucko AD, Hunt BJ, Kidd SL, Hom R. Randomized, double-blind, multicenter comparison of oral cefditoren 200 or 400 mg BID with either cefuroxime 250 mg BID or cefadroxil 500 mg BID for the treatment of uncomplicated skin and skin-structure infections. Clin Ther. 2002; 7. 24(7):1134–1147. PMID: 12182257.
Article24. Granizo JJ, Gimenez MJ, Barberan J, Coronel P, Gimeno M, Aguilar L. Efficacy of cefditoren in the treatment of upper respiratory tract infections: a pooled analysis of six clinical trials. Rev Esp Quimioter. 2008; 3. 21(1):14–21. PMID: 18443928.25. Granizo JJ, Gimenez MJ, Barberan J, Coronel P, Gimeno M, Aguilar L. The efficacy of cefditoren pivoxil in the treatment of lower respiratory tract infections, with a focus on the per-pathogen bacteriologic response in infections caused by Streptococcus pneumoniae and Haemophilus influenzae: a pooled analysis of seven clinical trials. Clin Ther. 2006; 12. 28(12):2061–2069. PMID: 17296462.26. Granizo JJ, Aguilar L, Gimenez MJ, Coronel P, Gimeno M, Prieto J. Safety profile of cefditoren: a pooled analysis of data from clinical trials in community-acquired respiratory tract infections. Rev Esp Quimioter. 2009; 6. 22(2):57–61. PMID: 19544097.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In Vitro Antimicrobial Activity of Cefditoren pivoxil, an Oral Cephalosporin, against Major Clinical Isolates
- A Randomized, Double-blinded, Open Label Study of the Efficacy and Safety of Cefcapene Pivoxil and Amoxicillin, Clavulanate in Acute Presumed Bacterial Rhinosinusitis
- Efficacy of Cefcapene Pivoxil for Empirical Therapy of Acute Uncomplicated Cystitis
- Diagnosis and treatment of acute rhinosinusitis in children
- Comparison of Doses of Doxapram in the Treatment of Postanesthetic Shivering