Three-Dimensional Printing: Basic Principles and Applications in Medicine and Radiology
- Affiliations
-
- 1Biomedical Engineering Research Center, Asan Institute of Life Science, Asan Medical Center, Seoul 05505, Korea.
- 2Department of Radiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea.
- 3Department of Cardiology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea.
- 4Department of Health Screening and Promotion Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea.
- 5Department of Urology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea.
- 6Department of Thoracic and Cardiovascular Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea.
- 7Department of Neurology, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea.
- 8POSTECH Biotech Center, Pohang University of Science and Technology, Pohang 37673, Korea.
- 9Department of Convergence Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul 05505, Korea. namkugkim@gmail.com
- KMID: 2360203
- DOI: http://doi.org/10.3348/kjr.2016.17.2.182
Abstract
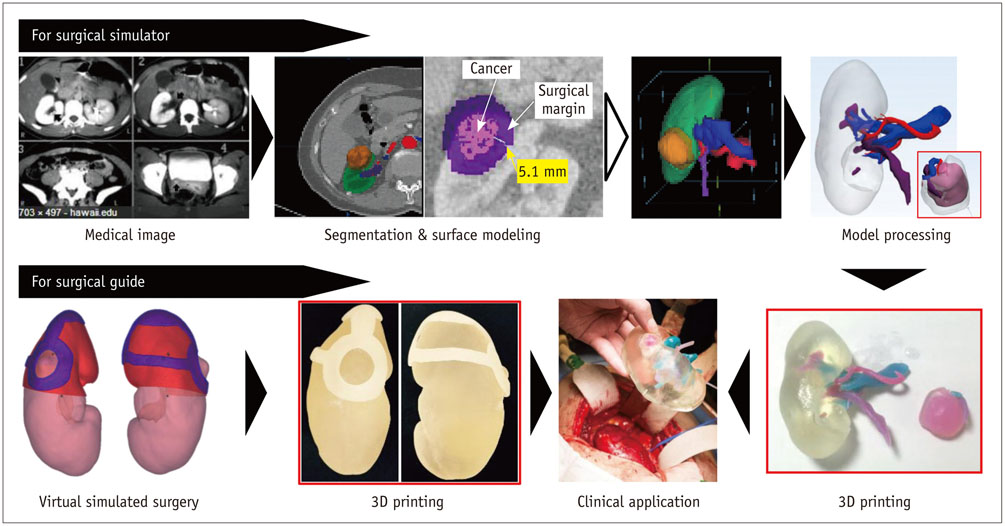
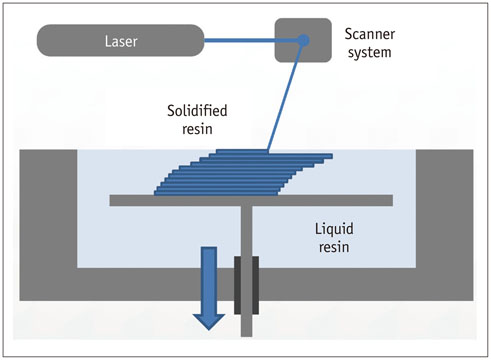
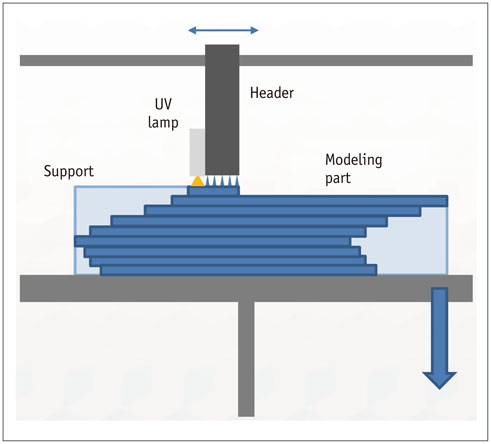
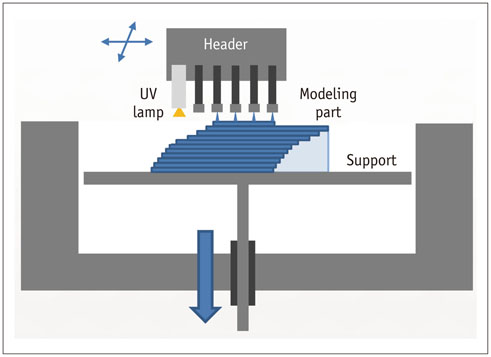
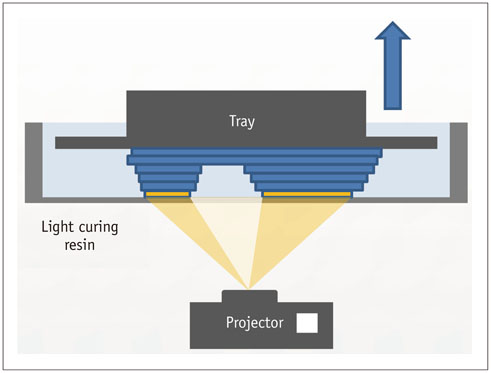
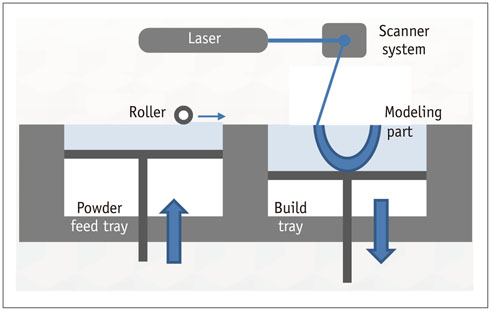
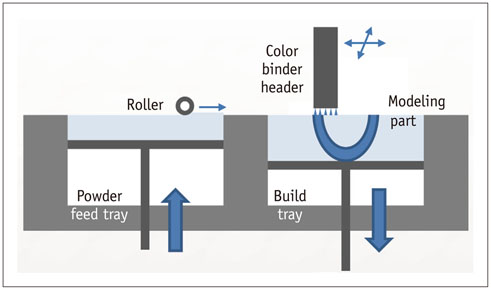
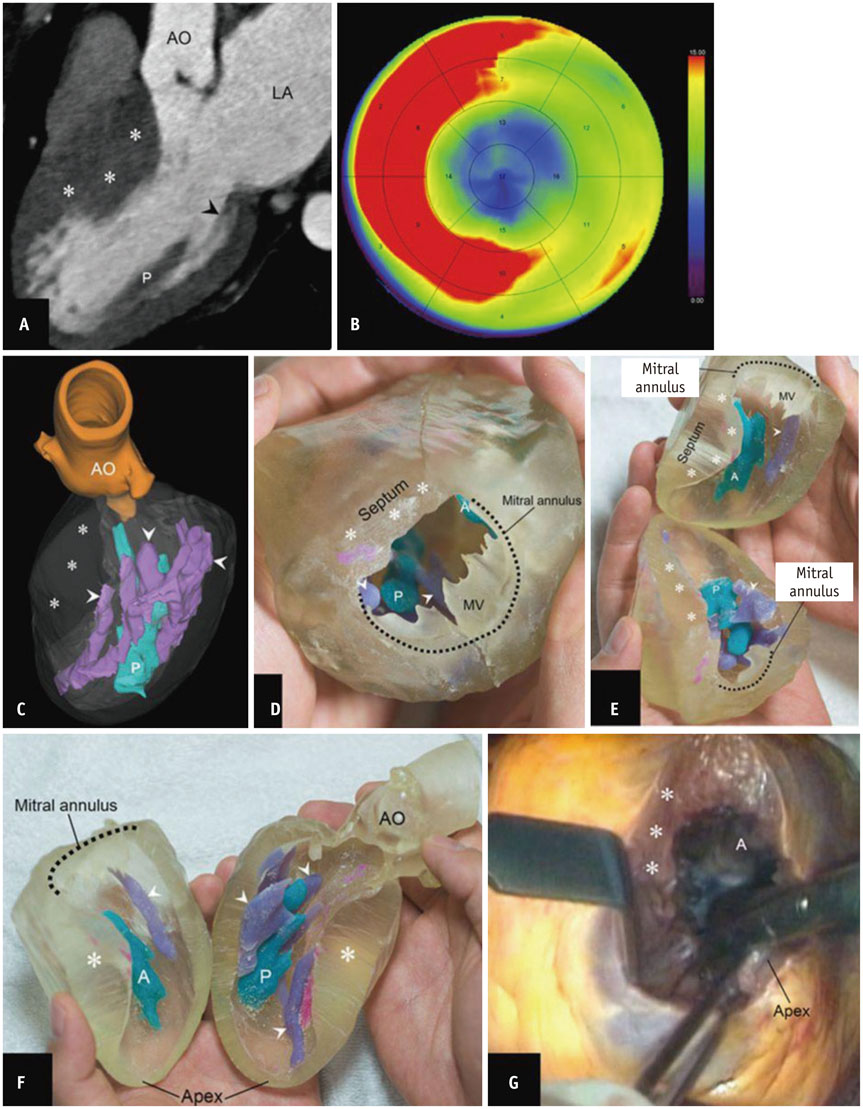
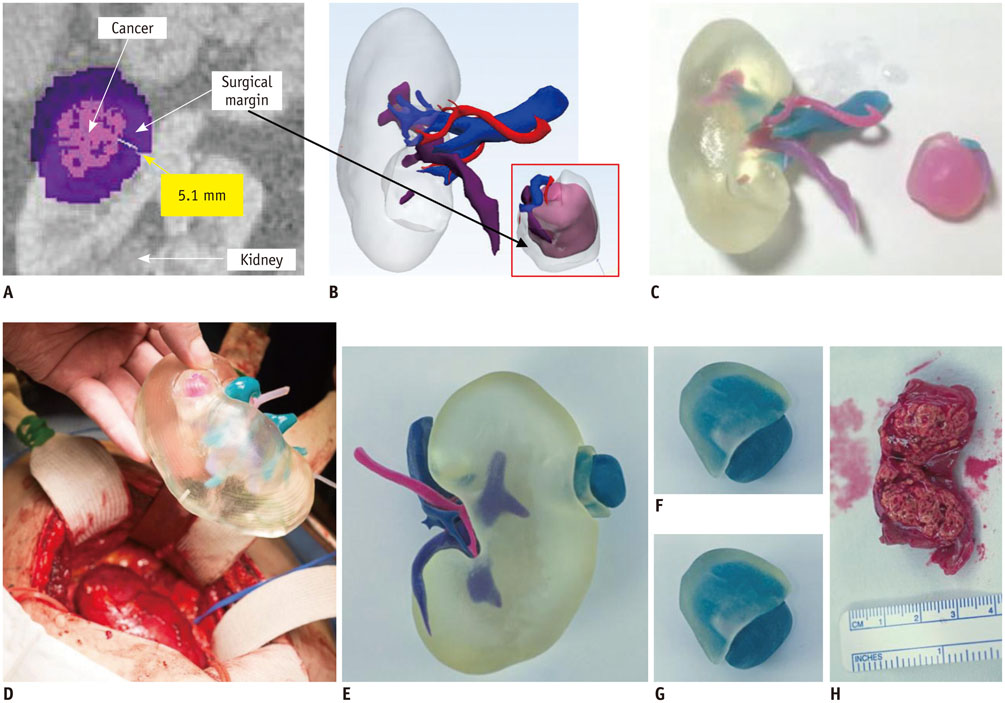
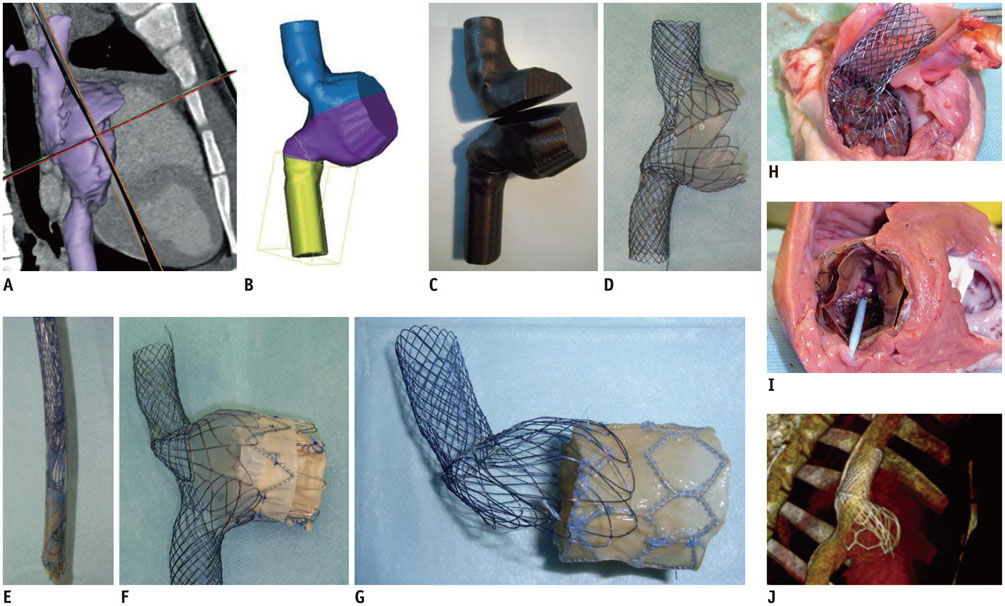
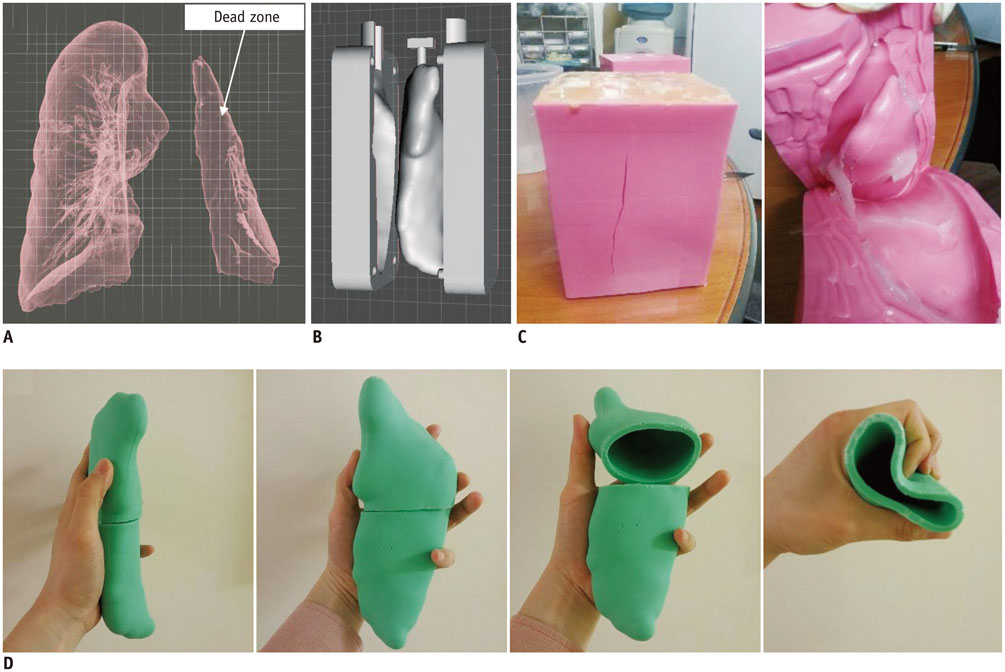
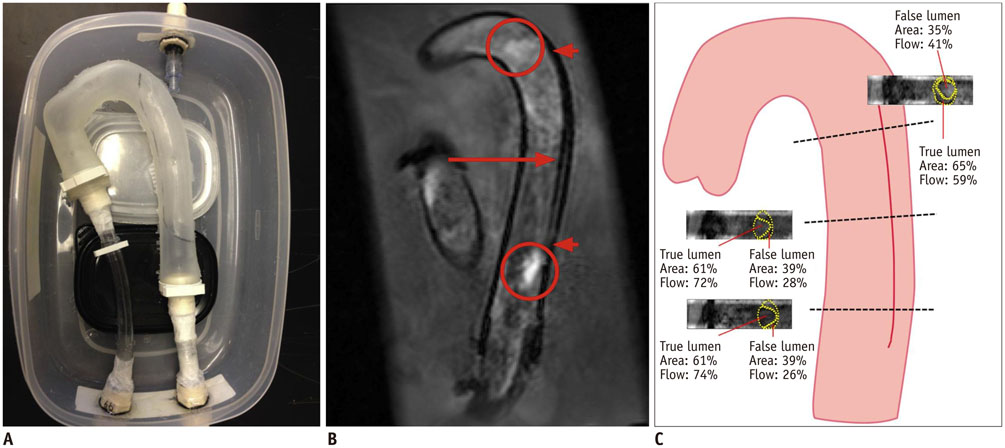
- The advent of three-dimensional printing (3DP) technology has enabled the creation of a tangible and complex 3D object that goes beyond a simple 3D-shaded visualization on a flat monitor. Since the early 2000s, 3DP machines have been used only in hard tissue applications. Recently developed multi-materials for 3DP have been used extensively for a variety of medical applications, such as personalized surgical planning and guidance, customized implants, biomedical research, and preclinical education. In this review article, we discuss the 3D reconstruction process, touching on medical imaging, and various 3DP systems applicable to medicine. In addition, the 3DP medical applications using multi-materials are introduced, as well as our recent results.
Keyword
MeSH Terms
Figure
Cited by 4 articles
-
Advanced Medical Use of Three-Dimensional Imaging in Congenital Heart Disease: Augmented Reality, Mixed Reality, Virtual Reality, and Three-Dimensional Printing
Hyun Woo Goo, Sang Joon Park, Shi-Joon Yoo
Korean J Radiol. 2020;21(2):133-145. doi: 10.3348/kjr.2019.0625.Three Dimensional Printing Technique and Its Application to Bone Tumor Surgery
Hyun Guy Kang, Jong Woong Park, Dae Woo Park
J Korean Orthop Assoc. 2018;53(6):466-477. doi: 10.4055/jkoa.2018.53.6.466.Age of Data in Contemporary Research Articles Published in Representative General Radiology Journals
Ji Hun Kang, Dong Hwan Kim, Seong Ho Park, Jung Hwan Baek
Korean J Radiol. 2018;19(6):1172-1178. doi: 10.3348/kjr.2018.19.6.1172.3D-Printed Disease Models for Neurosurgical Planning, Simulation, and Training
Chul-Kee Park
J Korean Neurosurg Soc. 2022;65(4):489-498. doi: 10.3340/jkns.2021.0235.
Reference
-
1. Kido T, Kurata A, Higashino H, Sugawara Y, Okayama H, Higaki J, et al. Cardiac imaging using 256-detector row four-dimensional CT: preliminary clinical report. Radiat Med. 2007; 25:38–44.2. Meaney JF, Goyen M. Recent advances in contrast-enhanced magnetic resonance angiography. Eur Radiol. 2007; 17:Suppl 2. B2–B6.3. Doi K. Diagnostic imaging over the last 50 years: research and development in medical imaging science and technology. Phys Med Biol. 2006; 51:R5–R27.4. Kirchgeorg MA, Prokop M. Increasing spiral CT benefits with postprocessing applications. Eur J Radiol. 1998; 28:39–54.5. McGurk M, Amis AA, Potamianos P, Goodger NM. Rapid prototyping techniques for anatomical modelling in medicine. Ann R Coll Surg Engl. 1997; 79:169–174.6. Wong KV, Hernandez A. A review of additive manufacturing. ISRN Mechanical Engineering. 2012 Jun 17. DOI: 10.5402/2012/208760. [Epub].7. Rengier F, Mehndiratta A, von Tengg-Kobligk H, Zechmann CM, Unterhinninghofen R, Kauczor HU, et al. 3D printing based on imaging data: review of medical applications. Int J Comput Assist Radiol Surg. 2010; 5:335–341.8. Gross BC, Erkal JL, Lockwood SY, Chen C, Spence DM. Evaluation of 3D printing and its potential impact on biotechnology and the chemical sciences. Anal Chem. 2014; 86:3240–3253.9. Michalski MH, Ross JS. The shape of things to come: 3D printing in medicine. JAMA. 2014; 312:2213–2214.10. Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nat Biotechnol. 2014; 32:773–785.11. Lorensen WE, Cline HE. Marching cubes: a high resolution 3D surface construction algorithm. SIGGRAPH Comput Graphics. 1987; 21:163–169.12. Tiede U, Höehne KH, Bomans M, Pommert A, Riemer M, Wiebecke G. Investigation of medical 3D-rendering algorithms. Comput Graphics Appl. 1990; 10:41–53.13. Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006; 31:1116–1128.14. Shattuck DW, Leahy RM. BrainSuite: an automated cortical surface identification tool. Med Image Anal. 2002; 6:129–142.15. Schroeder WJ, Zarge JA, Lorensen WE. Decimation of triangle meshes. SIGGRAPH Comput Graphics. 1992; 26:65–70.16. Field DA. Laplacian smoothing and Delaunay triangulations. Commun Appl Numer Methods. 1988; 4:709–712.17. Hinton E, Campbell JS. Local and global smoothing of discontinuous finite element functions using a least squares method. Int J Numer Method Eng. 1974; 8:461–480.18. Raphael O, Hervé R. Clinical applications of rapid prototyping models in cranio-maxillofacial surgery. In : Hoque M, editor. Advanced Applications of rapid prototyping technology in modern engineering. Rijeka, Croatia: InTech;2011.19. Mankovich NJ, Samson D, Pratt W, Lew D, Beumer J 3rd. Surgical planning using three-dimensional imaging and computer modeling. Otolaryngol Clin North Am. 1994; 27:875–889.20. Choi JY, Choi JH, Kim NK, Kim Y, Lee JK, Kim MK, et al. Analysis of errors in medical rapid prototyping models. Int J Oral Maxillofac Surg. 2002; 31:23–32.21. Chang PS, Parker TH, Patrick CW Jr, Miller MJ. The accuracy of stereolithography in planning craniofacial bone replacement. J Craniofac Surg. 2003; 14:164–170.22. Schicho K, Figl M, Seemann R, Ewers R, Lambrecht JT, Wagner A, et al. Accuracy of treatment planning based on stereolithography in computer assisted surgery. Med Phys. 2006; 33:3408–3417.23. Ibrahim D, Broilo TL, Heitz C, de Oliveira MG, de Oliveira HW, Nobre SM, et al. Dimensional error of selective laser sintering, three-dimensional printing and PolyJet models in the reproduction of mandibular anatomy. J Craniomaxillofac Surg. 2009; 37:167–173.24. Silva DN, Gerhardt de Oliveira M, Meurer E, Meurer MI, Lopes da Silva JV, Santa-Bárbara A. Dimensional error in selective laser sintering and 3D-printing of models for craniomaxillary anatomy reconstruction. J Craniomaxillofac Surg. 2008; 36:443–449.25. Shellabear M, Nyrhilä O. DMLS-Development history and state of the art. In : Proceedings of the 4th LANE; 2004 Sep 21-24; Erlangen, Germany. Bamberg: Meisenbach-Verlag;2004.26. Khaing MW, Fuh JY, Lu L. Direct metal laser sintering for rapid tooling: processing and characterization of EOS parts. J Mat Proc Tech. 2001; 113:269–272.27. Ohtani T, Kusumoto N, Wakabayashi K, Yamada S, Nakamura T, Kumazawa Y, et al. Application of haptic device to implant dentistry--accuracy verification of drilling into a pig bone. Dent Mater J. 2009; 28:75–81.28. Müller A, Krishnan KG, Uhl E, Mast G. The application of rapid prototyping techniques in cranial reconstruction and preoperative planning in neurosurgery. J Craniofac Surg. 2003; 14:899–914.29. Poukens J, Haex J, Riediger D. The use of rapid prototyping in the preoperative planning of distraction osteogenesis of the cranio-maxillofacial skeleton. Comput Aided Surg. 2003; 8:146–154.30. Wagner JD, Baack B, Brown GA, Kelly J. Rapid 3-dimensional prototyping for surgical repair of maxillofacial fractures: a technical note. J Oral Maxillofac Surg. 2004; 62:898–901.31. Faber J, Berto PM, Quaresma M. Rapid prototyping as a tool for diagnosis and treatment planning for maxillary canine impaction. Am J Orthod Dentofacial Orthop. 2006; 129:583–589.32. Mavili ME, Canter HI, Saglam-Aydinatay B, Kamaci S, Kocadereli I. Use of three-dimensional medical modeling methods for precise planning of orthognathic surgery. J Craniofac Surg. 2007; 18:740–747.33. D'Urso PS, Earwaker WJ, Barker TM, Redmond MJ, Thompson RG, Effeney DJ, et al. Custom cranioplasty using stereolithography and acrylic. Br J Plast Surg. 2000; 53:200–204.34. Paiva WS, Amorim R, Bezerra DA, Masini M. Aplication of the stereolithography technique in complex spine surgery. Arq Neuropsiquiatr. 2007; 65:443–445.35. Armillotta A, Bonhoeffer P, Dubini G, Ferragina S, Migliavacca F, Sala G, et al. Use of rapid prototyping models in the planning of percutaneous pulmonary valved stent implantation. Proc Inst Mech Eng H. 2007; 221:407–416.36. Kim MS, Hansgen AR, Wink O, Quaife RA, Carroll JD. Rapid prototyping: a new tool in understanding and treating structural heart disease. Circulation. 2008; 117:2388–2394.37. Wurm G, Tomancok B, Pogady P, Holl K, Trenkler J. Cerebrovascular stereolithographic biomodeling for aneurysm surgery. Technical note. J Neurosurg. 2004; 100:139–145.38. Giesel FL, Hart AR, Hahn HK, Wignall E, Rengier F, Talanow R, et al. 3D reconstructions of the cerebral ventricles and volume quantification in children with brain malformations. Acad Radiol. 2009; 16:610–617.39. Guarino J, Tennyson S, McCain G, Bond L, Shea K, King H. Rapid prototyping technology for surgeries of the pediatric spine and pelvis: benefits analysis. J Pediatr Orthop. 2007; 27:955–960.40. Hurson C, Tansey A, O'Donnchadha B, Nicholson P, Rice J, McElwain J. Rapid prototyping in the assessment, classification and preoperative planning of acetabular fractures. Injury. 2007; 38:1158–1162.41. Hiramatsu H, Yamaguchi H, Nimi S, Ono H. [Rapid prototyping of the larynx for laryngeal frame work surgery]. Nihon Jibiinkoka Gakkai Kaiho. 2004; 107:949–955.42. Mahmood F, Owais K, Taylor C, Montealegre-Gallegos M, Manning W, Matyal R, et al. Three-dimensional printing of mitral valve using echocardiographic data. JACC Cardiovasc Imaging. 2015; 8:227–229.43. Yang DH, Kang JW, Kim N, Song JK, Lee JW, Lim TH. Myocardial 3-dimensional printing for septal myectomy guidance in a patient with obstructive hypertrophic cardiomyopathy. Circulation. 2015; 132:300–301.44. He J, Li D, Lu B, Wang Z, Tao Z. Custom fabrication of composite tibial hemi-knee joint combining CAD/CAE/CAM techniques. Proc Inst Mech Eng H. 2006; 220:823–830.45. Wang Z, Teng Y, Li D. [Fabrication of custom-made artificial semi-knee joint based on rapid prototyping technique: computer-assisted design and manufacturing]. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2004; 18:347–351.46. Lee MY, Chang CC, Ku YC. New layer-based imaging and rapid prototyping techniques for computer-aided design and manufacture of custom dental restoration. J Med Eng Technol. 2008; 32:83–90.47. Dai KR, Yan MN, Zhu ZA, Sun YH. Computer-aided custom-made hemipelvic prosthesis used in extensive pelvic lesions. J Arthroplasty. 2007; 22:981–986.48. Harrysson OL, Hosni YA, Nayfeh JF. Custom-designed orthopedic implants evaluated using finite element analysis of patient-specific computed tomography data: femoralcomponent case study. BMC Musculoskelet Disord. 2007; 8:91.49. Stevens B, Yang Y, Mohandas A, Stucker B, Nguyen KT. A review of materials, fabrication methods, and strategies used to enhance bone regeneration in engineered bone tissues. J Biomed Mater Res B Appl Biomater. 2008; 85:573–582.50. Peltola SM, Melchels FP, Grijpma DW, Kellomäki M. A review of rapid prototyping techniques for tissue engineering purposes. Ann Med. 2008; 40:268–280.51. Amerini A, Hatam N, Malasa M, Pott D, Tewarie L, Isfort P, et al. A personalized approach to interventional treatment of tricuspid regurgitation: experiences from an acute animal study. Interact Cardiovasc Thorac Surg. 2014; 19:414–418.52. Griffith LG, Naughton G. Tissue engineering--current challenges and expanding opportunities. Science. 2002; 295:1009–1014.53. Melchels FP, Domingos MA, Klein TJ, Malda J, Bartolo PJ, Hutmacher DW. Additive manufacturing of tissues and organs. Prog Polym Sci. 2012; 37:1079–1104.54. Huh D, Matthews BD, Mammoto A, Montoya-Zavala M, Hsin HY, Ingber DE. Reconstituting organ-level lung functions on a chip. Science. 2010; 328:1662–1668.55. Huh D, Hamilton GA, Ingber DE. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011; 21:745–754.56. Chung SK, Son YR, Shin SJ, Kim SK. Nasal airflow during respiratory cycle. Am J Rhinol. 2006; 20:379–384.57. Tek P, Chiganos TC, Mohammed JS, Eddington DT, Fall CP, Ifft P, et al. Rapid prototyping for neuroscience and neural engineering. J Neurosci Methods. 2008; 172:263–269.58. Canstein C, Cachot P, Faust A, Stalder AF, Bock J, Frydrychowicz A, et al. 3D MR flow analysis in realistic rapid-prototyping model systems of the thoracic aorta: comparison with in vivo data and computational fluid dynamics in identical vessel geometries. Magn Reson Med. 2008; 59:535–546.59. Dhir V, Itoi T, Fockens P, Perez-Miranda M, Khashab MA, Seo DW, et al. Novel ex vivo model for hands-on teaching of and training in EUS-guided biliary drainage: creation of "Mumbai EUS" stereolithography/3D printing bile duct prototype (with videos). Gastrointest Endosc. 2015; 81:440–446.60. de Zélicourt D, Pekkan K, Kitajima H, Frakes D, Yoganathan AP. Single-step stereolithography of complex anatomical models for optical flow measurements. J Biomech Eng. 2005; 127:204–207.61. Giesel FL, Mehndiratta A, von Tengg-Kobligk H, Schaeffer A, Teh K, Hoffman EA, et al. Rapid prototyping raw models on the basis of high resolution computed tomography lung data for respiratory flow dynamics. Acad Radiol. 2009; 16:495–498.62. Sulaiman A, Boussel L, Taconnet F, Serfaty JM, Alsaid H, Attia C, et al. In vitro non-rigid life-size model of aortic arch aneurysm for endovascular prosthesis assessment. Eur J Cardiothorac Surg. 2008; 33:53–57.63. Birjiniuk J, Ruddy JM, Iffrig E, Henry TS, Leshnower BG, Oshinski JN, et al. Development and testing of a silicone in vitro model of descending aortic dissection. J Surg Res. 2015; 198:502–507.64. Veeraswamy RK, Birjiniuk J, Ruddy JM, Timmins L, Oshinski JN, Ku DN. PC42. Phase-contrast magnetic resonance imaging reveals novel fluid dynamics in a patient-derived silicone model of descending thoracic aortic dissection. J Vasc Surg. 2015; 61:128S–129S.65. Kim BJ, Kwon SU. Perforator infarction immediately distal to the stenosis of parental artery: is it hemodynamic? J Stroke Cerebrovasc Dis. 2014; 23:1991–1993.66. Suzuki M, Ogawa Y, Kawano A, Hagiwara A, Yamaguchi H, Ono H. Rapid prototyping of temporal bone for surgical training and medical education. Acta Otolaryngol. 2004; 124:400–402.67. Bruyère F, Leroux C, Brunereau L, Lermusiaux P. Rapid prototyping model for percutaneous nephrolithotomy training. J Endourol. 2008; 22:91–96.68. Kalejs M, von Segesser LK. Rapid prototyping of compliant human aortic roots for assessment of valved stents. Interact Cardiovasc Thorac Surg. 2009; 8:182–186.69. Waran V, Narayanan V, Karuppiah R, Owen SL, Aziz T. Utility of multimaterial 3D printers in creating models with pathological entities to enhance the training experience of neurosurgeons. J Neurosurg. 2014; 120:489–492.70. Narayanan V, Narayanan P, Rajagopalan R, Karuppiah R, Rahman ZA, Wormald PJ, et al. Endoscopic skull base training using 3D printed models with pre-existing pathology. Eur Arch Otorhinolaryngol. 2015; 272:753–757.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Application of Three-Dimensional Printing in the Fracture Management
- A Review of Current Clinical Applications of Three-Dimensional Printing in Spine Surgery
- Clinical Applications of Three-Dimensional Printing in Cardiovascular Disease
- Medical Applications of 3D Printing and Standardization Issues
- Applications of Three-Dimensional Printing in Cardiovascular Surgery: A Case-Based Review