Brain Tumor Res Treat.
2016 Oct;4(2):100-106. 10.14791/btrt.2016.4.2.100.
White Matter Change Revealed by Diffusion Tensor Imaging in Gliomas
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Hospital, Seoul, Korea. chungc@snu.ac.kr
- 2Neuroscience Research Institute, Seoul National University Medical Research Center, Seoul, Korea.
- 3Clinical Research Institute, Seoul National University Hospital, Seoul, Korea.
- 4Department of Biomedical Engineering, Hanyang University, Seoul, Korea.
- KMID: 2356977
- DOI: http://doi.org/10.14791/btrt.2016.4.2.100
Abstract
- BACKGROUND
Tumor-related white matter change is detected at late stages with magnetic resonance imaging (MRI), when mass effect or prominent edema is present. We analyzed if diffusion tensor imaging (DTI) white matter change earlier than conventional MRI.
METHODS
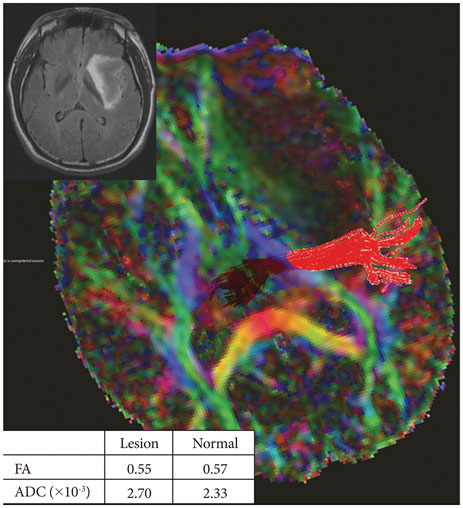
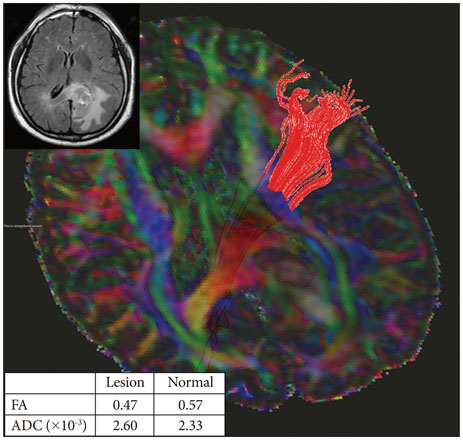
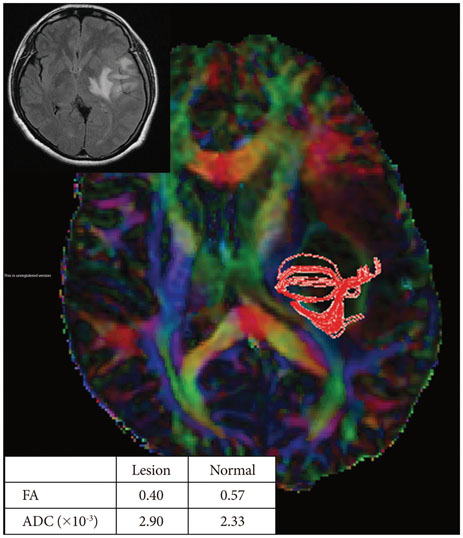
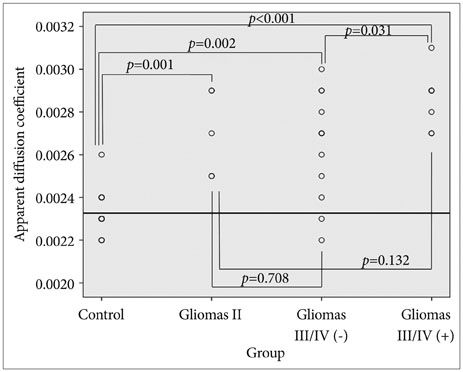
Twenty-six patients with gliomas (World Health Organization grade II, 5; grade III, 12; and grade IV, 9) within 2 cm from the posterior limb of the internal capsule (IC) were studied. Fifteen normal adults were enrolled as controls. Fluid attenuation inversion recovery MRI showed a high signal change at the posterior limb of the IC (HSIC) in 9 patients with grade III or IV gliomas. We classified the gliomas as WHO grade II (gliomas II), grade III or IV without HSIC [gliomas III/IV(-)] and grade III or IV with HSIC [gliomas III/IV(+)], as an indicator of the increase in the severity of the white matter changes. Fractional anisotropy (FA) and apparent diffusion coefficients (ADC) were calculated for the pyramidal tract. Tumor progression along pyramidal tract was evaluated by follow-up MRI in 16 patients at 40±18 months.
RESULTS
FA showed no significant difference between gliomas II and control (p=0.694), but was lower in gliomas III/IV(-) and gliomas III/IV(+) (p<0.001). ADCs were higher in gliomas II, gliomas III/IV(-) and gliomas III/IV(+) than control (p<0.001). Tumor progression was detected in 2/16 patients.
CONCLUSION
DTI detected white matter changes that appeared to be normal in MRI. ADC changed even in low grade glioma, indicating ADC may be a better parameter for the early detection of white matter change.
MeSH Terms
Figure
Reference
-
1. Price SJ, Jena R, Burnet NG, Carpenter TA, Pickard JD, Gillard JH. Predicting patterns of glioma recurrence using diffusion tensor imaging. Eur Radiol. 2007; 17:1675–1684.
Article2. Price SJ, Jena R, Burnet NG, et al. Improved delineation of glioma margins and regions of infiltration with the use of diffusion tensor imaging: an image-guided biopsy study. AJNR Am J Neuroradiol. 2006; 27:1969–1974.3. Schlüter M, Stieltjes B, Hahn HK, Rexilius J, Konrad-verse O, Peitgen HO. Detection of tumour infiltration in axonal fibre bundles using diffusion tensor imaging. Int J Med Robot. 2005; 1:80–86.
Article4. Johnson PC, Hunt SJ, Drayer BP. Human cerebral gliomas: correlation of postmortem MR imaging and neuropathologic findings. Radiology. 1989; 170(1 Pt 1):211–217.
Article5. Mabray MC, Barajas RF Jr, Cha S. Modern brain tumor imaging. Brain Tumor Res Treat. 2015; 3:8–23.
Article6. Giese A, Kluwe L, Laube B, Meissner H, Berens ME, Westphal M. Migration of human glioma cells on myelin. Neurosurgery. 1996; 38:755–764.
Article7. Nilsson D, Rutka JT, Snead OC 3rd, Raybaud CR, Widjaja E. Preserved structural integrity of white matter adjacent to low-grade tumors. Childs Nerv Syst. 2008; 24:313–320.
Article8. Watanabe M, Tanaka R, Takeda N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology. 1992; 34:463–469.
Article9. Akai H, Mori H, Aoki S, et al. Diffusion tensor tractography of gliomatosis cerebri: fiber tracking through the tumor. J Comput Assist Tomogr. 2005; 29:127–129.10. Duffau H, Thiebaut de Schotten M, Mandonnet E. White matter functional connectivity as an additional landmark for dominant temporal lobectomy. J Neurol Neurosurg Psychiatry. 2008; 79:492–495.
Article11. Han BS, Ahn SH, Jang SH. Cortical reorganization demonstrated by diffusion tensor tractography analyzed using functional MRI activation. NeuroRehabilitation. 2008; 23:171–174.
Article12. Kim CH, Chung CK, Kim JS, Jahng TA, Lee JH, Song IC. Use of diffusion tensor imaging to evaluate weakness. J Neurosurg. 2007; 106:111–118.
Article13. Lee HY, Na DG, Song IC, et al. Diffusion-tensor imaging for glioma grading at 3-T magnetic resonance imaging: analysis of fractional anisotropy and mean diffusivity. J Comput Assist Tomogr. 2008; 32:298–303.
Article14. Bastin ME, Carpenter TK, Armitage PA, Sinha S, Wardlaw JM, Whittle IR. Effects of dexamethasone on cerebral perfusion and water diffusion in patients with high-grade glioma. AJNR Am J Neuroradiol. 2006; 27:402–408.15. Sinha S, Bastin ME, Wardlaw JM, Armitage PA, Whittle IR. Effects of dexamethasone on peritumoural oedematous brain: a DT-MRI study. J Neurol Neurosurg Psychiatry. 2004; 75:1632–1635.
Article16. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971; 9:97–113.
Article17. Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999; 45:265–269.
Article18. Concha L, Beaulieu C, Gross DW. Bilateral limbic diffusion abnormalities in unilateral temporal lobe epilepsy. Ann Neurol. 2005; 57:188–196.
Article19. Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002; 17:77–94.
Article20. Kim CH, Kim JH, Chung CK, Kim JS, Lee JM, Lee SK. Localization of broca's area using functional MR imaging: quantitative evaluation of paradigms. J Korean Neurosurg Soc. 2009; 45:219–223.
Article21. Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980; 30:907–911.
Article22. Silbergeld DL, Chicoine MR. Isolation and characterization of human malignant glioma cells from histologically normal brain. J Neurosurg. 1997; 86:525–531.
Article23. Halperin EC, Bentel G, Heinz ER, Burger PC. Radiation therapy treatment planning in supratentorial glioblastoma multiforme: an analysis based on post mortem topographic anatomy with CT correlations. Int J Radiat Oncol Biol Phys. 1989; 17:1347–1350.
Article24. Beppu T, Inoue T, Shibata Y, et al. Fractional anisotropy value by diffusion tensor magnetic resonance imaging as a predictor of cell density and proliferation activity of glioblastomas. Surg Neurol. 2005; 63:56–61. discussion 61.
Article25. Goebell E, Fiehler J, Ding XQ, et al. Disarrangement of fiber tracts and decline of neuronal density correlate in glioma patients--a combined diffusion tensor imaging and 1H-MR spectroscopy study. AJNR Am J Neuroradiol. 2006; 27:1426–1431.26. Price SJ, Burnet NG, Donovan T, et al. Diffusion tensor imaging of brain tumours at 3T: a potential tool for assessing white matter tract invasion? Clin Radiol. 2003; 58:455–462.
Article27. Morita K, Matsuzawa H, Fujii Y, Tanaka R, Kwee IL, Nakada T. Diffusion tensor analysis of peritumoral edema using lambda chart analysis indicative of the heterogeneity of the microstructure within edema. J Neurosurg. 2005; 102:336–341.
Article28. Tropine A, Vucurevic G, Delani P, et al. Contribution of diffusion tensor imaging to delineation of gliomas and glioblastomas. J Magn Reson Imaging. 2004; 20:905–912.
Article29. Provenzale JM, McGraw P, Mhatre P, Guo AC, Delong D. Peritumoral brain regions in gliomas and meningiomas: investigation with isotropic diffusion-weighted MR imaging and diffusion-tensor MR imaging. Radiology. 2004; 232:451–460.
Article30. Sundgren PC, Fan X, Weybright P, et al. Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging. 2006; 24:1131–1142.
Article31. Toh CH, Wong AM, Wei KC, Ng SH, Wong HF, Wan YL. Peritumoral edema of meningiomas and metastatic brain tumors: differences in diffusion characteristics evaluated with diffusion-tensor MR imaging. Neuroradiology. 2007; 49:489–494.
Article32. Stadlbauer A, Ganslandt O, Buslei R, et al. Gliomas: histopathologic evaluation of changes in directionality and magnitude of water diffusion at diffusion-tensor MR imaging. Radiology. 2006; 240:803–810.
Article33. Stadlbauer A, Nimsky C, Buslei R, et al. Diffusion tensor imaging and optimized fiber tracking in glioma patients: histopathologic evaluation of tumor-invaded white matter structures. Neuroimage. 2007; 34:949–956.
Article34. Lunsford LD, Martinez AJ, Latchaw RE. Magnetic resonance imaging does not define tumor boundaries. Acta Radiol Suppl. 1986; 369:154–156.35. Schonberg T, Pianka P, Hendler T, Pasternak O, Assaf Y. Characterization of displaced white matter by brain tumors using combined DTI and fMRI. Neuroimage. 2006; 30:1100–1111.
Article36. Smits M, Vernooij MW, Wielopolski PA, Vincent AJ, Houston GC, van der Lugt A. Incorporating functional MR imaging into diffusion tensor tractography in the preoperative assessment of the corticospinal tract in patients with brain tumors. AJNR Am J Neuroradiol. 2007; 28:1354–1361.
Article37. Kunimatsu A, Itoh D, Nakata Y, et al. Utilization of diffusion tensor tractography in combination with spatial normalization to assess involvement of the corticospinal tract in capsular/pericapsular stroke: feasibility and clinical implications. J Magn Reson Imaging. 2007; 26:1399–1404.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Usefulness of Diffusion-tensor MR Imaging (DTI) in the Grading of Gliomas
- Diffusion Tensor Imaging Findings of White Matter Changes in First Episode Schizophrenia: A Systematic Review
- Brain Diffusion Tensor MR Imaging
- Alteration of White Matter Integrity in Dyslexic Children: Case-Control Study
- Diffusion Tensor Imaging: Exploring the Motor Networks and Clinical Applications