J Korean Med Sci.
2016 Dec;31(12):1914-1921. 10.3346/jkms.2016.31.12.1914.
Once-Daily OROS Hydromorphone for Management of Cancer Pain: an Open-Label, Multi-Center, Non-Interventional Study
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Hwasun Hospital, Hwasun, Korea. droij@chonnam.ac.kr
- 2Department of Thoracic and Cardiovascular surgery, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 3Department of Radiation Oncology, Chonnam National University Hwasun Hospital, Hwasun, Korea.
- 4Department of Internal Medicine, Pusan National University Hospital, Busan, Korea.
- KMID: 2355619
- DOI: http://doi.org/10.3346/jkms.2016.31.12.1914
Abstract
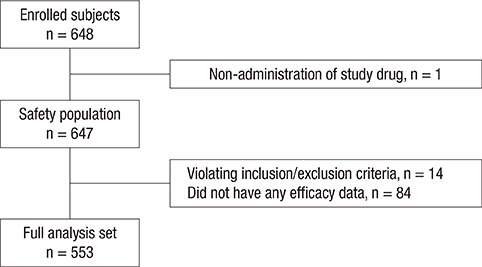
- Extended-release osmotic extended-release oral delivery system (OROS) hydromorphone is a strong synthetic opioid designed to maintain a constant blood concentration by once daily dosing. The objective of this observational study was to investigate the clinical usefulness of OROS hydromorphone in patients with cancer pain of moderate to severe intensity. Patients with cancer pain who required strong opioids were administered with OROS hydromorphone for 4 weeks. We assessed changes in pain intensity using a numerical rating scale (NRS) as well as levels of sleep disturbance, breakthrough pain, end-of-dose failure, patient satisfaction, and overall assessment of drug effectiveness based on investigator evaluation. Of the 648 enrolled patients, 553 patients were included in the full analysis set. The mean pain intensity was significantly decreased from the NRS value of 5.07 ± 1.99 to 2.75 ± 1.94 (mean % change of 42.13 ± 46.53, P < 0.001). The degree of sleep disturbance significantly improved (mean NRS change of 1.61 ± 2.57, P < 0.001), and the incidence of breakthrough pain was significantly decreased (mean NRS change of 1.22 ± 2.30, P < 0.001). The experience of end-of-dose failure also significantly decreased from 4.60 ± 1.75 to 3.93 ± 1.70, P = 0.007). The patient satisfaction rate was 72.7%, and 72.9% of investigators evaluated the study drug as effective. OROS hydromorphone was an effective and tolerable agent for cancer pain management. It effectively lowered pain intensity as well as improved sleep disturbance, breakthrough pain, and end-of-dose failure (Identifier: NCT 01273454).
Keyword
MeSH Terms
Figure
Reference
-
1. Ventafridda V, Caraceni A, Gamba A. Field-testing of the WHO guidelines for cancer pain relief: summary report of demonstration projects. In : Foley KM, Bonica JJ, Ventafridda V, editors. Proceedings of the Second International Congress on Cancer Pain. Vol. 16. Advances in Pain Research and Therapy. New York, NY: Raven Press, Ltd.;1990. p. 451–464.2. Schug SA, Zech D, Dörr U. Cancer pain management according to WHO analgesic guidelines. J Pain Symptom Manage. 1990; 5:27–32.3. Portenoy RK, Lesage P. Management of cancer pain. Lancet. 1999; 353:1695–1700.4. World Health Organization. Cancer Pain Relief. 2nd ed. Geneva: World Health Organization;1996.5. Cherny NJ, Chang V, Frager G, Ingham JM, Tiseo PJ, Popp B, Portenoy RK, Foley KM. Opioid pharmacotherapy in the management of cancer pain: a survey of strategies used by pain physicians for the selection of analgesic drugs and routes of administration. Cancer. 1995; 76:1283–1293.6. Chaplan SR, Duncan SR, Brodsky JB, Brose WG. Morphine and hydromorphone epidural analgesia. A prospective, randomized comparison. Anesthesiology. 1992; 77:1090–1094.7. Goodarzi M. Comparison of epidural morphine, hydromorphone and fentanyl for postoperative pain control in children undergoing orthopaedic surgery. Paediatr Anaesth. 1999; 9:419–422.8. Wallace M, Moulin DE, Rauck RL, Khanna S, Tudor IC, Skowronski R, Thipphawong J. Long-term safety, tolerability, and efficacy of OROS hydromorphone in patients with chronic pain. J Opioid Manag. 2009; 5:97–105.9. Pigni A, Brunelli C, Caraceni A. The role of hydromorphone in cancer pain treatment: a systematic review. Palliat Med. 2011; 25:471–477.10. Kumar MG, Lin S. Hydromorphone in the management of cancer-related pain: an update on routes of administration and dosage forms. J Pharm Pharm Sci. 2007; 10:504–518.11. Bruera E, Sloan P, Mount B, Scott J, Suarez-Almazor M. A randomized, double-blind, double-dummy, crossover trial comparing the safety and efficacy of oral sustained-release hydromorphone with immediate-release hydromorphone in patients with cancer pain. Canadian Palliative Care Clinical Trials Group. J Clin Oncol. 1996; 14:1713–1717.12. Ferrell B, Wisdom C, Wenzl C, Brown J. Effects of controlled-released morphine on quality of life for cancer pain. Oncol Nurs Forum. 1989; 16:521–526.13. Palangio M, Northfelt DW, Portenoy RK, Brookoff D, Doyle RT Jr, Dornseif BE, Damask MC. Dose conversion and titration with a novel, once-daily, OROS osmotic technology, extended-release hydromorphone formulation in the treatment of chronic malignant or nonmalignant pain. J Pain Symptom Manage. 2002; 23:355–368.14. Drover DR, Angst MS, Valle M, Ramaswamy B, Naidu S, Stanski DR, Verotta D. Input characteristics and bioavailability after administration of immediate and a new extended-release formulation of hydromorphone in healthy volunteers. Anesthesiology. 2002; 97:827–836.15. Angst MS, Drover DR, Lötsch J, Ramaswamy B, Naidu S, Wada DR, Stanski DR. Pharmacodynamics of orally administered sustained- release hydromorphone in humans. Anesthesiology. 2001; 94:63–73.16. Verma RK, Krishna DM, Garg S. Formulation aspects in the development of osmotically controlled oral drug delivery systems. J Control Release. 2002; 79:7–27.17. Bass DM, Prevo M, Waxman DS. Gastrointestinal safety of an extended-release, nondeformable, oral dosage form (OROS®): a retrospective study. Drug Saf. 2002; 25:1021–1033.18. Turgeon J, Gröning R, Sathyan G, Thipphawong J, Richarz U. The pharmacokinetics of a long-acting OROS hydromorphone formulation. Expert Opin Drug Deliv. 2010; 7:137–144.19. Wallace M, Rauck RL, Moulin D, Thipphawong J, Khanna S, Tudor IC. Once-daily OROS hydromorphone for the management of chronic nonmalignant pain: a dose-conversion and titration study. Int J Clin Pract. 2007; 61:1671–1676.20. Goforth HW. Hydromorphone-OROS formulation. Expert Opin Pharmacother. 2010; 11:1207–1214.21. Shin SH, Lee HS, Kim YS, Choi YJ, Kim SH, Kwon HC, Oh SY, Kang JH, Sohn CH, Lee SM, et al. Clinical usefulness of hydromorphone-OROS in improving sleep disturbances in Korean cancer patients: a multicenter, prospective, open-label study. Cancer Res Treat. 2014; 46:331–338.22. Guay DR. Oral hydromorphone extended-release. Consult Pharm. 2010; 25:816–828.23. Hanna M, Thipphawong J. 118 Study Group. A randomized, double-blind comparison of OROS(R) hydromorphone and controlled-release morphine for the control of chronic cancer pain. BMC Palliat Care. 2008; 7:17.24. Hanna M, Tuca A, Thipphawong J. An open-label, 1-year extension study of the long-term safety and efficacy of once-daily OROS(R) hydromorphone in patients with chronic cancer pain. BMC Palliat Care. 2009; 8:14.25. Gardner-Nix J, Mercadante S. The role of OROS hydromorphone in the management of cancer pain. Pain Pract. 2010; 10:72–77.26. Han HS, Lee KH, Lee KH, Ryu JS, Kim YC, Park SW, Oh HS, Park KT, Kwon JH, Lee PB, et al. A prospective, open-label, multicenter study of the clinical efficacy of extended-release hydromorphone in treating cancer pain inadequately controlled by other analgesics. Support Care Cancer. 2014; 22:741–750.27. Song EK, Shim H, Han HS, Sun D, Lee SI, Kang MH, Lee K, Cho D, Cho IS, Park SY, et al. A prospective multicentre study to evaluate the efficacy and tolerability of osmotic release oral system (OROS) hydromorphone in opioid-naive cancer patients: results of the Korean South West Oncology Group study. Pain Res Manag. 2015; 20:293–299.28. Jonsson T, Christrup LL, Højsted J, Villesen HH, Albjerg TH, Ravn-Nielsen LV, Sjøgren P. Symptoms and side effects in chronic non-cancer pain: patient report vs. systematic assessment. Acta Anaesthesiol Scand. 2011; 55:69–74.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Tolerability of OROS Hydromorphone in Strong Opioid-Naive Patients: An Open Label, Prospective Study
- Clinical Usefulness of Hydromorphone-OROS in Improving Sleep Disturbances in Korean Cancer Patients: A Multicenter, Prospective, Open-Label Study
- Hydromorphone attenuates intercellular adhesion molecule-1 expressions induced by lipopolysaccharide on HCT-116 human colon cancer cells
- A Comparison of Hydromorphone-Bupivacaine and Fentanyl-Bupivacaine in Patient Controlled Epidural Analgesia after Thoracotomy
- Breakthrough Cancer Pain