Korean Circ J.
2016 Nov;46(6):811-820. 10.4070/kcj.2016.46.6.811.
Role of Soluble ST2 as a Marker for Rejection after Heart Transplant
- Affiliations
-
- 1Division of Cardiology, Cardiac and Vascular Center, Department of Medicine, Samsung Medical Center, Sungkyunkwan University, School of Medicine, Seoul, Korea. choijean5@gmail.com
- KMID: 2355457
- DOI: http://doi.org/10.4070/kcj.2016.46.6.811
Abstract
- BACKGROUND AND OBJECTIVES
Endomyocardial biopsy is obligatory during the first year after heart transplant (HTx) for the surveillance of acute rejection. Previous attempts using cardiac biomarkers for the detection of rejection failed to show enough evidence to substitute endomyocardial biopsy. Therefore, this study sought the possibility of using soluble ST2 (sST2), a novel cardiovascular marker, as a surrogate marker for acute allograft rejection after HTx.
SUBJECTS AND METHODS
A total of 494 blood samples acquired at the time of endomyocardial biopsy were analyzed in 67 HTx cases from September 2006 to August 2014. Significant rejection was defined as International Society of Heart and Lung Transplant (ISHLT) score ≥2R and humoral rejection accompanied by hemodynamic instability.
RESULTS
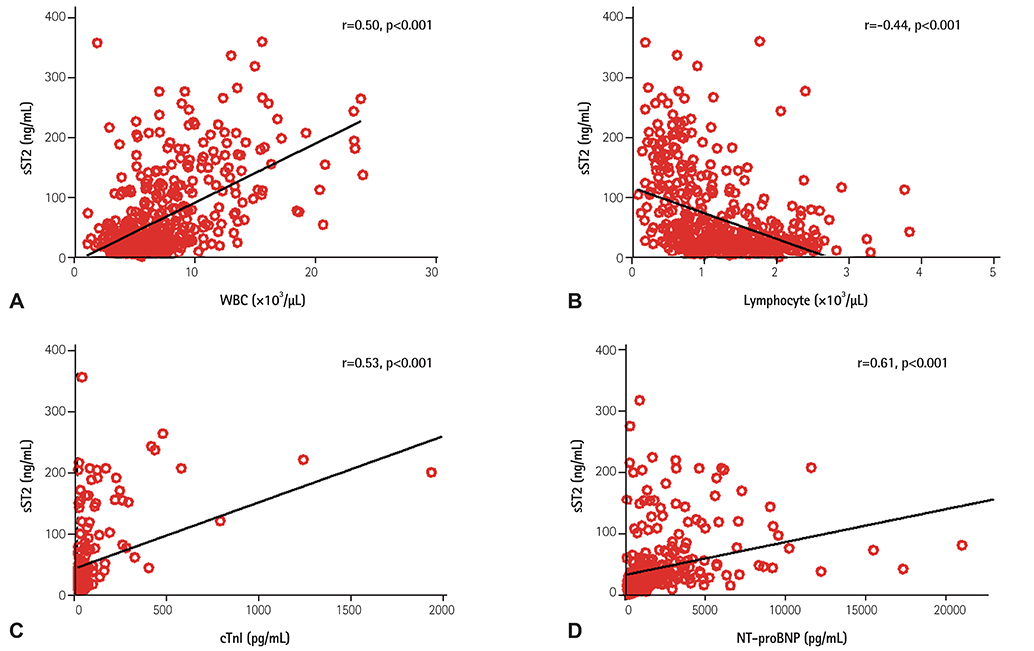
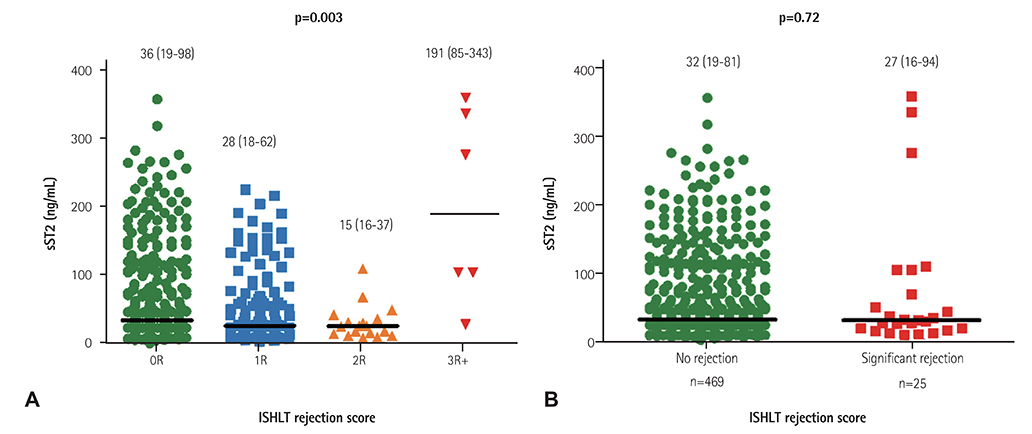
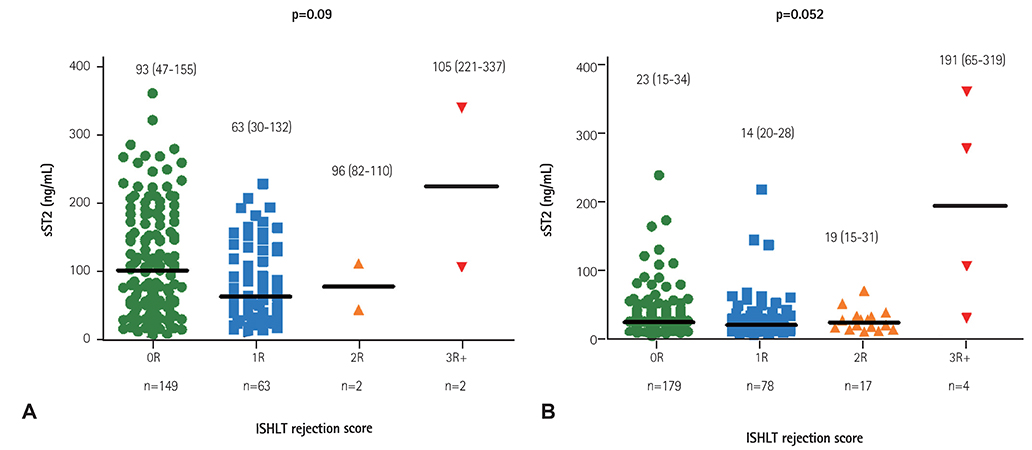
Twenty cases of HTx with 22 blood samples showed significant rejection in endomyocardial biopsy at 4.0 (2.0-9.0) months after HTx. The level of sST2 showed positive correlation with cardiac troponin I, and N-terminal pro-B-type natriuretic peptide (all p<0.001), and negative correlation with post-HTx months (p<0.001). The levels of sST2 according to the ISHLT scores were 36 (19-98), 28 (18-62), 15 (16-37), and 191 (85-343) ng/mL, consecutively 0R, 1R, 2R, and 3R+ (3R plus hemodynamically-unstable humoral rejection) (p=0.003). However, when we studied within-subject effects of sST2 using a mixed model, the sST2 level according to the predefined time point was not different according to the presence of significant rejection (p for interaction=0.94).
CONCLUSION
Although sST2 is known as a promising predictor for cardiovascular events, its role in HTx patients to predict acute allograft rejection seems to be limited.
MeSH Terms
Figure
Reference
-
1. Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report--2012. J Heart Lung Transplant. 2012; 31:1052–1064.2. Costanzo MR, Dipchand A, Starling R, et al. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010; 29:914–956.3. Nguyen V, Cantarovich M, Cecere R, Giannetti N. Tricuspid regurgitation after cardiac transplantation: how many biopsies are too many? J Heart Lung Transplant. 2005; 24:7 Suppl. S227–S231.4. Wong RC, Abrahams Z, Hanna M, et al. Tricuspid regurgitation after cardiac transplantation: an old problem revisited. J Heart Lung Transplant. 2008; 27:247–252.5. Marelli D, Esmailian F, Wong SY, et al. Tricuspid valve regurgitation after heart transplantation. J Thorac Cardiovasc Surg. 2009; 137:1557–1559.6. Battes LC, Caliskan K, Rizopoulos D, et al. Repeated measurements of NT-pro-B-type natriuretic peptide, troponin T or C-reactive protein do not predict future allograft rejection in heart transplant recipients. Transplantation. 2015; 99:580–585.7. Patel PC, Hill DA, Ayers CR, et al. High-sensitivity cardiac troponin I assay to screen for acute rejection in patients with heart transplant. Circ Heart Fail. 2014; 7:463–469.8. Ahn KT, Choi JO, Lee GY, Park HD, Jeon ES. Usefulness of high-sensitivity troponin I for the monitoring of subclinical acute cellular rejection after cardiac transplantation. Transplant Proc. 2015; 47:504–510.9. Januzzi JL Jr. ST2 as a cardiovascular risk biomarker: from the bench to the bedside. J Cardiovasc Transl Res. 2013; 6:493–500.10. Januzzi JL Jr, Rehman S, Mueller T, van Kimmenade RR, Lloyd-Jones DM. Importance of biomarkers for long-term mortality prediction in acutely dyspneic patients. Clin Chem. 2010; 56:1814–1821.11. Shimpo M, Morrow DA, Weinberg EO, et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004; 109:2186–2190.12. Wang TJ, Wollert KC, Larson MG, et al. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012; 126:1596–1604.13. Kim BS, Jeon ES. Soluble ST2; predictive biomarkers of clinical presentation and mechanical circulation support in fulminant myocarditis. Korean Circ J. 2014; 322:Abstract.14. Stewart S, Winters GL, Fishbein MC, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005; 24:1710–1720.15. Arora S, Gullestad L, Wergeland R, et al. Probrain natriuretic peptide and C-reactive protein as markers of acute rejection, allograft vasculopathy, and mortality in heart transplantation. Transplantation. 2007; 83:1308–1315.16. Pascual-Figal DA, Garrido IP, Blanco R, et al. Soluble ST2 is a marker for acute cardiac allograft rejection. Ann Thorac Surg. 2011; 92:2118–2124.17. Mathews LR, Lott JM, Isse K, et al. Elevated ST2 distinguishes incidences of pediatric heart and small bowel transplant rejection. Am J Transplant. 2016; 16:938–950.18. Kakkar R, Lee RT. The IL-33/ST2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008; 7:827–840.19. Ciccone MM, Cortese F, Gesualdo M, et al. A novel cardiac biomarker: ST2: a review. Molecules. 2013; 18:15314–15328.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Heart Transplant Rejection Treated with Plasmapheresis
- Elevated levels of soluble ST2 but not galectin-3 are associated with increased risk of mortality in hemodialysis patients
- Establishing Reference Intervals for Soluble ST2 Assay in a Korean Population
- Acute Vascular Rejection 11 Months after Heart Transplantation
- Diagnosis of renal transplant rejection: Banff classification and beyond