Korean Circ J.
2016 Nov;46(6):784-790. 10.4070/kcj.2016.46.6.784.
The Association between Whole Blood Viscosity and Coronary Collateral Circulation in Patients with Chronic Total Occlusion
- Affiliations
-
- 1Department of Cardiology, Türkiye Yüksek Ihtisas Training and Research Hospital, Ankara, Turkey. mehmetserkancetin@gmail.com
- KMID: 2355453
- DOI: http://doi.org/10.4070/kcj.2016.46.6.784
Abstract
- BACKGROUND AND OBJECTIVES
Coronary collateral circulation (CCC) has been attributed as inborn bypass mechanisms supporting ischemic myocardium. Various factors have been postulated in CCC. Whole blood viscosity (WBV) has been an underappreciated entity despite close relationships between multiple cardiovascular diseases. WBV can be calculated with a validated equation from hematocrit and total plasma protein levels for a low and high shear rate. On the grounds, we aimed to evaluate the association between WBV and CCC in patients with chronic total occlusion.
SUBJECTS AND METHODS
A total of 371 patients diagnosed as having at least one major, chronic total occluded coronary artery were included. 197 patients with good CCC (Rentrop 2 and 3) composed the patient group. The poor collateral group consisted of 174 patients (Rentrop grade 0 and 1).
RESULTS
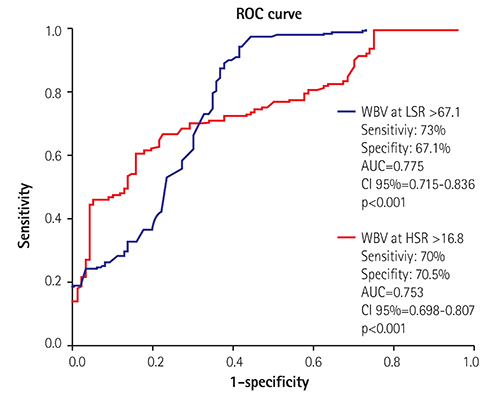
Patients with poor CCC had higher WBV values for a low-shear rate (LSR) (69.5±8.7 vs. 60.1±9.8, p<0.001) and high-shear rate (HSR) (17.0±2.0 vs. 16.4±1.8, p<0.001) than the good collateral group. Correlation analysis demonstrated a significant negative correlation between the grade of CCC and WBV for LSR (β=0.597, p<0.001) and HSR (β=0.494, p<0.001). WBV for LSR (β=0.476, p<0.001) and HSR (β=0.407, p<0.001) had a significant correlation with the synergy between percutaneous coronary intervention with taxus and cardiac surgery (SYNTAX) score. A multivariate analysis showed that the WBV for both shear rates were independent risk factors of poor CCC (WBV at LSR, OR: 1.362 CI 95%: 1.095-1.741 p<0.001 and WBV at HSR, 1.251 CI 95%: 1.180-1.347 p<0.001).
CONCLUSION
WBV has been demonstrated as the overlooked predictor of poor coronary collateralization. WBV seemed to be associated with microvascular perfusion and angiogenesis process impairing CCC development.
MeSH Terms
Figure
Cited by 2 articles
-
Sex-based Approach for the Clinical Impact of the Increased Hemoglobin on Incident AF in the General Population
In-Soo Kim, Byoung Kwon Lee, Pil-Sung Yang, Boyoung Joung, Jong-Youn Kim
Korean Circ J. 2020;50(12):1095-1110. doi: 10.4070/kcj.2020.0412.Establishing Reference Intervals of Whole Blood Viscosity in a Korean Population Using a Cone-Plate Viscometer
Mikyoung Park, Hanah Kim, Hee-Won Moon, Mina Hur, Yeo-Min Yun
Lab Med Online. 2021;11(3):162-170. doi: 10.47429/lmo.2021.11.3.162.
Reference
-
1. Cohen M, Rentrop KP. Limitation of myocardial ischemia by collateral circulation during sudden controlled coronary artery occlusion in human subjects: a prospective study. Circulation. 1986; 74:469–476.2. Kilian JG, Keech A, Adams MR, Celermajer DS. Coronary collateralization: determinants of adequate distal vessel filling after arterial occlusion. Coron Artery Dis. 2002; 13:155–159.3. van Royen N, Piek JJ, Buschmann I, Hoefer I, Voskuil M, Schaper W. Stimulation of arteriogenesis; a new concept for the treatment of arterial occlusive disease. Cardiovasc Res. 2001; 49:543–553.4. Papaioannou TG, Stefanadis C. Vascular wall shear stress: basic principles and methods. Hellenic J Cardiol. 2005; 46:9–15.5. Sloop G, Holsworth RE Jr, Weidman JJ, St Cyr JA. The role of chronic hyperviscosity in vascular disease. Ther Adv Cardiovasc Dis. 2015; 9:19–25.6. Cho YI, Cho DJ, Rosenson RS. Endothelial shear stress and blood viscosity in peripheral arterial disease. Curr Atheroscler Rep. 2014; 16:404.7. de Simone G, Devereux RB, Chien S, Alderman MH, Atlas SA, Laragh JH. Relation of blood viscosity to demographic and physiologic variables and to cardiovascular risk factors in apparently normal adults. Circulation. 1990; 81:107–117.8. Shah PB. Management of coronary chronic total occlusion. Circulation. 2011; 123:1780–1784.9. Rentrop KP, Cohen M, Blanke H, Phillips RA. Changes in collateral channel filling immediately after controlled coronary artery occlusion by an angioplasty balloon in human subjects. J Am Coll Cardiol. 1985; 5:587–592.10. Sloop GD, Garber DW. The effects of low-density lipoprotein and high-density lipoprotein on blood viscosity correlate with their association with risk of atherosclerosis in humans. Clin Sci (Lond). 1997; 92:473–479.11. Blann A, Bignell A, McCollum C. von Willebrand factor, fibrinogen and other plasma proteins as determinants of plasma viscosity. Atherosclerosis. 1998; 139:317–322.12. Muggeo M, Calabrò A, Businaro V, Moghetti P, Padovan D, Crepaldi G. Correlation of metabolic and hemorrheological parameters in diabetes and hyperlipidemia. Ric Clin Lab. 1983; 13:Suppl 3. 165–179.13. Wasilewski J, Turczyński B, Słowińska L, Kowalik V, Osadnik T, Poloński L. Haemorheological factors and myocardial reperfusion in patients with ST-elevation myocardial infarction undergoing primary coronary intervention. Kardiol Pol. 2007; 65:778–785. discussion 786-7.14. Empen K, Geiss HC, Lehrke M, Otto C, Schwandt P, Parhofer KG. Effect of atorvastatin on lipid parameters, LDL subtype distribution, hemorrheological parameters and adhesion molecule concentrations in patients with hypertriglyceridemia. Nutr Metab Cardiovasc Dis. 2003; 13:87–92.15. Mellwig KP, Baller D, Schmidt HK, et al. Myocardial perfusion under H.E.L.P.-apheresis. Objectification by PET. Z Kardiol. 2003; 92:Suppl 3. III30–III37.16. Arntz HR, Perchalla G, Roll D, Heitz J, Schäfer JH, Schröder R. Blood rheology in acute myocardial infarction: effects of high-dose i.v. streptokinase compared to placebo. Eur Heart J. 1992; 13:275–280.17. Mohandas N, Chasis JA, Shohet SB. The influence of membrane skeleton on red cell deformability, membrane material properties, and shape. Semin Hematol. 1983; 20:225–242.18. Hansen JF. Coronary collateral circulation: clinical significance and influence on survival in patients with coronary artery occlusion. Am Heart J. 1989; 117:290–295.19. Nacar AB, Erayman A, Kurt M, et al. The relationship between coronary collateral circulation and neutrophil/lymphocyte ratio in patients with coronary chronic total occlusion. Med Princ Pract. 2015; 24:65–69.20. Börekçi A, Gür M, Şeker T, et al. Coronary collateral circulation in patients with chronic coronary total occlusion; its relationship with cardiac risk markers and SYNTAX score. Perfusion. 2015; 30:457–464.21. Yaylali YT, Susam I, Demir E, et al. Increased red blood cell deformability and decreased aggregation as potential adaptive mechanisms in the slow coronary flow phenomenon. Coron Artery Dis. 2013; 24:11–15.22. Soulis JV, Farmakis TM, Giannoglou GD, et al. Molecular viscosity in the normal left coronary arterial tree. Is it related to atherosclerosis? Angiology. 2006; 57:33–40.23. Biro GP, Beresford-Kroeger D, Hendry P. Early deleterious hemorheologic changes following acute experimental coronary occlusion and salutary antihyperviscosity effect of hemodilution with stroma-free hemoglobin. Am Heart J. 1982; 103:870–878.24. Biro GP, Beresford-Kroeger D. The effect of hemodilution with stroma-free hemoglobin and dextran on collateral perfusion of ischemic myocardium in the dog. Am Heart J. 1980; 99:64–75.25. Doi H, Kaburaki M, Inoue H, Suzumura K, Narita H. Protective effect of TA-993, a novel therapeutic agent for peripheral circulatory insufficiency, on skeletal muscle fatigue in a rat model of hindlimb ischemia. Jpn J Pharmacol. 2000; 83:73–81.26. van Gaal WJ, Ponnuthurai FA, Selvanayagam J, et al. The Syntax score predicts peri-procedural myocardial necrosis during percutaneous coronary intervention. Int J Cardiol. 2009; 135:60–65.27. Kyriakides ZS, Kremastinos DT, Michelakakis NA, Matsakas EP, Demovelis T, Toutouzas PK. Coronary collateral circulation in coronary artery disease and systemic hypertension. Am J Cardiol. 1991; 67:687–690.28. Junker R, Heinrich J, Ulbrich H, et al. Relationship between plasma viscosity and the severity of coronary heart disease. Arterioscler Thromb Vasc Biol. 1998; 18:870–875.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Development of Collateral Circulation in Patients with Total Occlusion of Coronary Artery and its Clinical Significance
- Assessment of Myocardial Collateral Blood Flow with Contrast Echocardiography
- Hybrid Coronary Artery Revascularization for Takayasu Arteritis with Major Visceral Collateral Circulation from the Left Internal Thoracic Artery
- Recanalization of a Coronary Chronic Total Occlusion by a Retrograde Approach Using Ipsilateral Double Guiding Catheters
- A Retrograde Approach to Coronary Ostial Stenosis after a Bentall Procedure in a Patient with Behcet's Disease