Korean Circ J.
2015 Sep;45(5):351-356. 10.4070/kcj.2015.45.5.351.
Assessment of Myocardial Collateral Blood Flow with Contrast Echocardiography
- Affiliations
-
- 1Knight Cardiovascular Institute, Oregon Health & Science University, Portland, Oregon, USA. kauls@ohsu.edu
- KMID: 2223796
- DOI: http://doi.org/10.4070/kcj.2015.45.5.351
Abstract
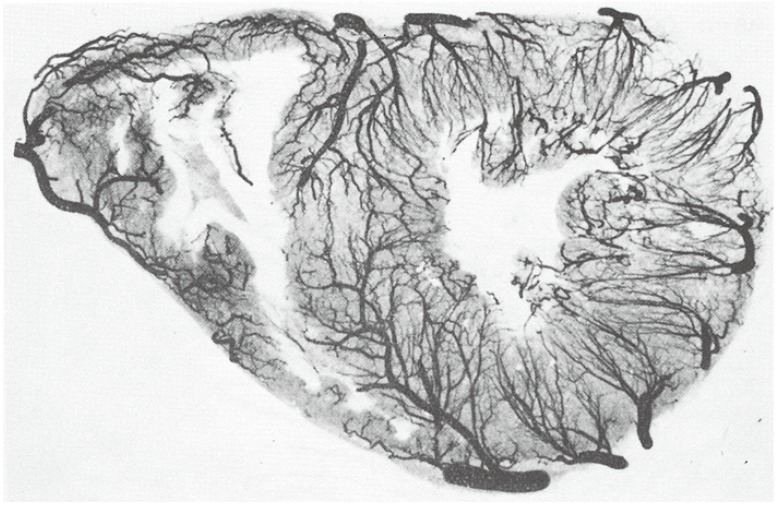
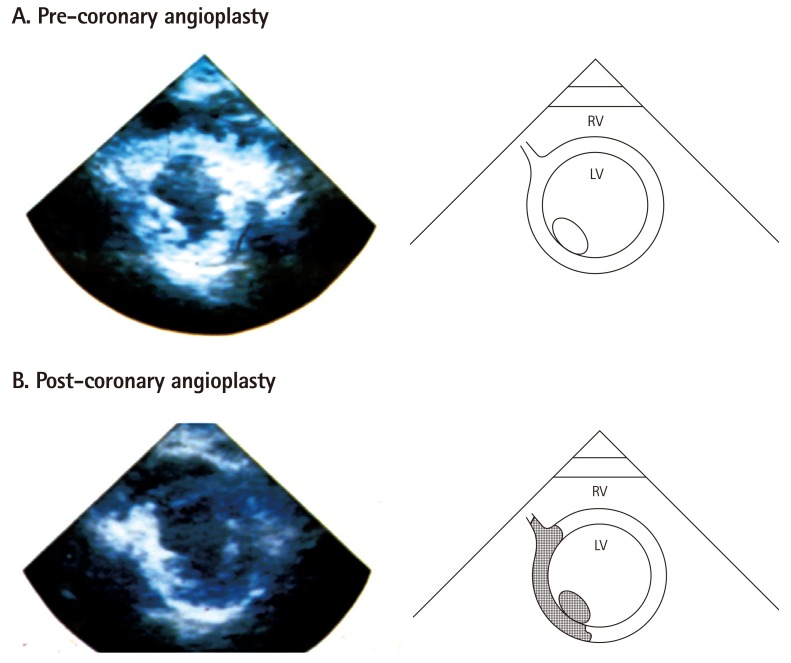
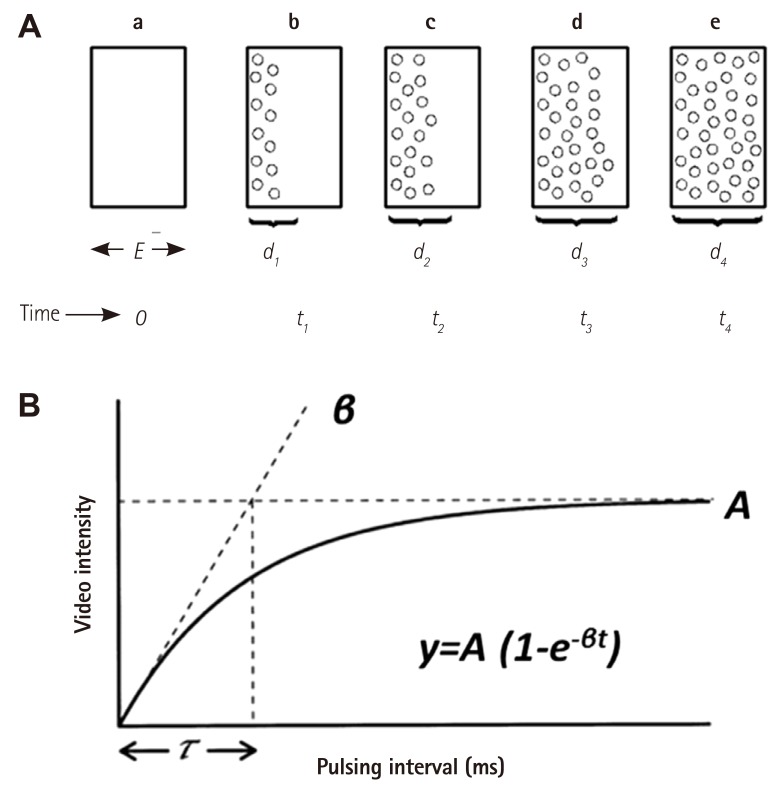
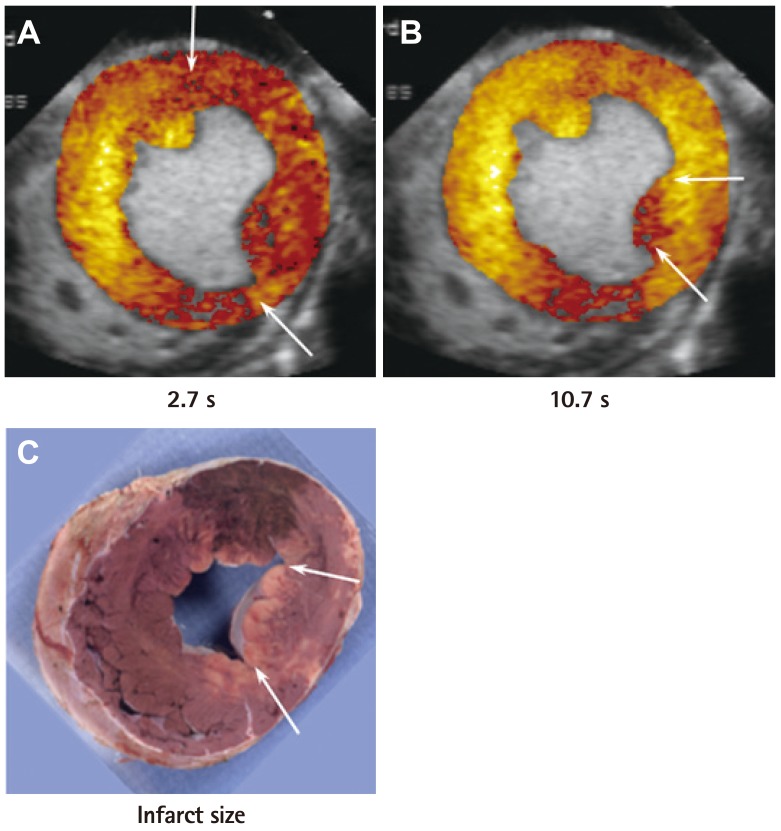
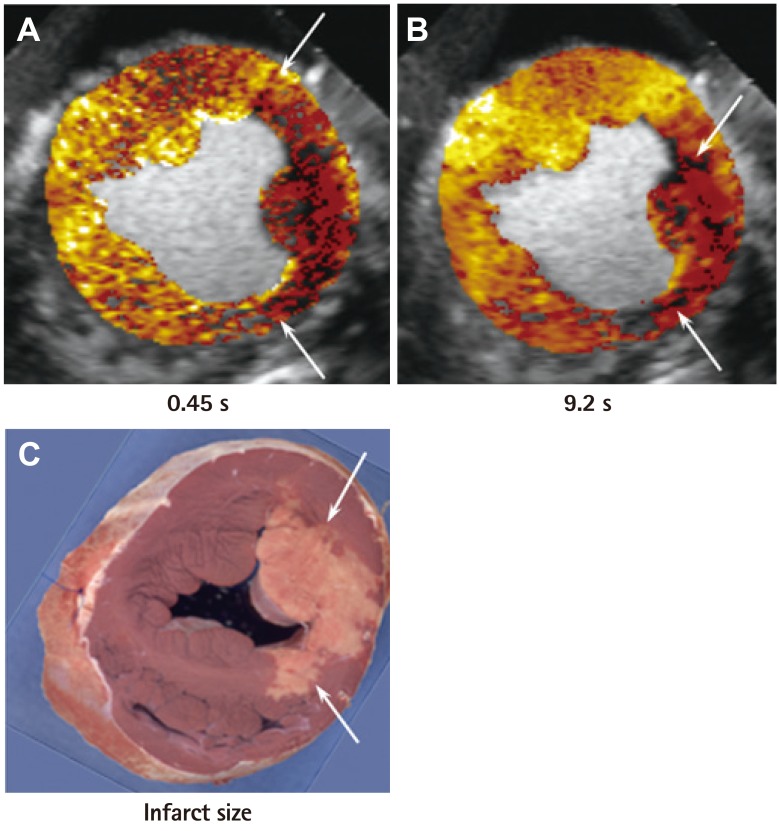
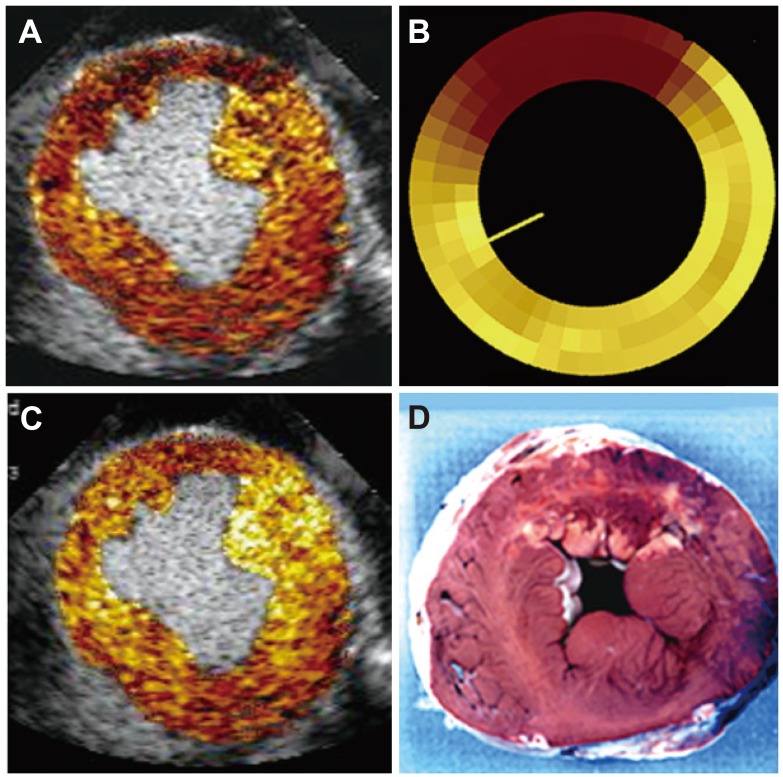
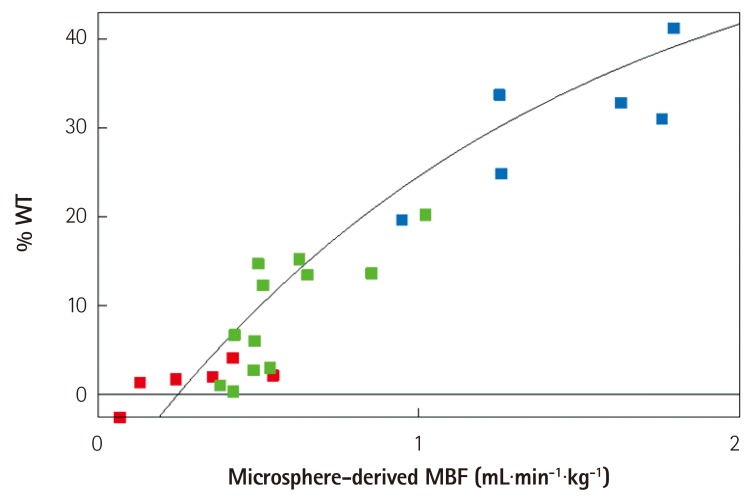
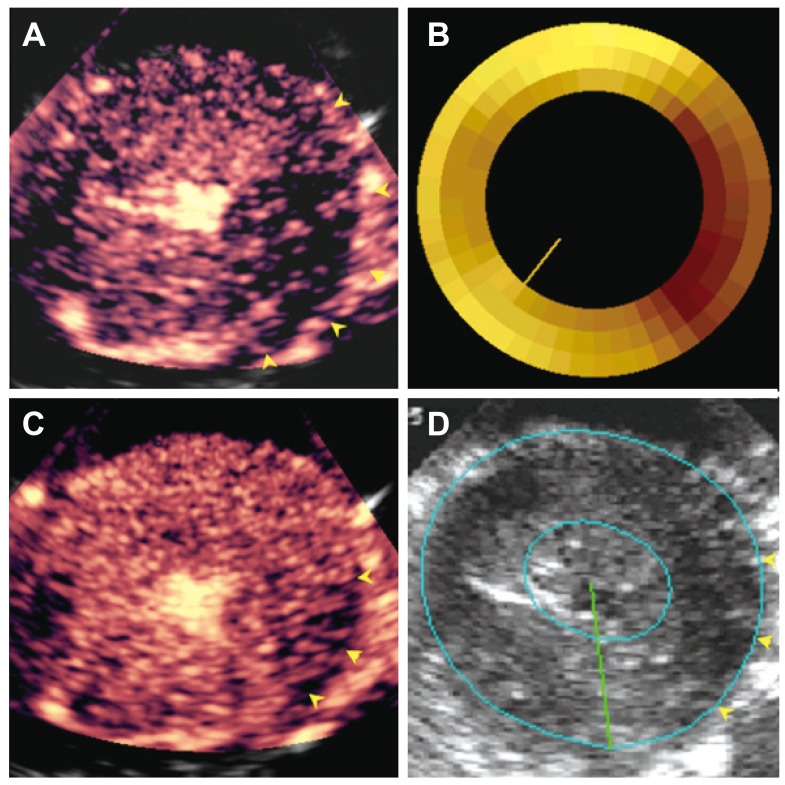
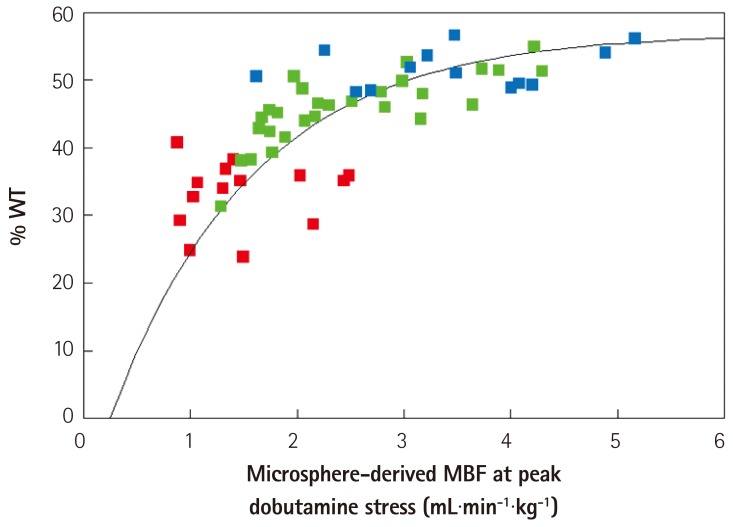
- Humans have pre-formed collateral vessels that enlarge with ischemia. In addition, new vessels can be formed within ischemic zones from pre-formed endocardial arcades of vessels providing rich collateral flow. Collateral flow under resting conditions (if >25% of normal) is enough to maintain myocardial viability, but may be insufficient to prevent myocardial ischemia under stress. Coronary angiography is a poor tool for collateral flow assessment. Myocardial contrast echocardiography is arguably the gold standard for experimental and clinical measurement of collateral flow. This review describes several experimental and clinical studies that highlight the importance of the collateral circulation in coronary artery disease.
MeSH Terms
Figure
Reference
-
1. Lower R. Tractatus de corde item de motu et colore sanguinis et chli in eum transitu. KJ Franklin. Ealry Science in Oxford, Vol. 9. Oxford: Oxford University Press;1932. p. 13.2. Gross L, Kugel MA. The arterial blood vascular distribution to the left and right ventricles of the human heart. Am Heart J. 1933; 9:165–177.3. Fulton WF. Anastomotic enlargement and ischemic myocardial damage. Br Heart J. 1964; 26:1–15. PMID: 14106121.4. Baroldi G, Scomazzoni G. Coronary circulation in the normal and pathological heart. Washington, D.C.: Armed Forces Institute of Pathology;1967.5. Schaper W. Collateral Circulation: past and present. Basic Res Cardiol. 2009; 104:5–21. PMID: 19101749.6. Cohen MV. Coronary collaterals: clinical & experimental observations. Mount Kisco, N.Y.: Futura Pub. Co.;1985.7. Kaul S. Myocardial contrast echocardiography: 15 years of research and development. Circulation. 1997; 96:3745–3760. PMID: 9396479.8. Kaul S. Myocardial contrast echocardiography: a 25-year retrospective. Circulation. 2008; 118:291–308. PMID: 18625905.9. Sabia PJ, Powers ER, Ragosta M, Sarembock IJ, Burwell LR, Kaul S. An association between collateral blood flow and myocardial viability in patients with recent myocardial infarction. N Engl J Med. 1992; 327:1825–1831. PMID: 1448120.10. Sabia PJ, Powers ER, Jayaweera AR, Ragosta M, Kaul S. Functional significance of collateral blood flow in patients with recent acute myocardial infarction. A study using myocardial contrast echocardiography. Circulation. 1992; 85:2080–2089. PMID: 1591827.11. Kaul S, Gillam LD, Weyman AE. Contrast echocardiography in acute myocardial ischemia. II. The effect of site of injection of contrast agent on the estimation of area at risk for necrosis after coronary occlusion. J Am Coll Cardiol. 1985; 6:825–830. PMID: 3897342.12. Kaul S, Glasheen WP, Oliner JD, Kelly P, Gascho JA. Relation between anterograde blood flow through a coronary artery and the perfusion bed it supplies: experimental and clinical implications. J Am Coll Cardiol. 1991; 17:1403–1413. PMID: 2016458.13. Kaul S, Pandian NG, Guerrero JL, Gillam LD, Okada RD, Weyman AE. Effects of selectively altering the collateral driving pressure on regional perfusion and function in the occluded coronary bed in the dog. Circ Res. 1987; 61:77–85. PMID: 3608113.14. Vernon SM, Camarano G, Kaul S, et al. Myocardial contrast echocardiography demonstrates that collateral flow can preserve myocardial function beyond a chronically occluded coronary artery. Am J Cardiol. 1996; 78:958–960. PMID: 8888677.15. Keller MW, Segal SS, Kaul S, Duling B. The behavior of sonicated albumin microbubbles within the microcirculation: a basis for their use during myocardial contrast echocardiography. Circ Res. 1989; 65:458–467. PMID: 2752551.16. Jayaweera AR, Edwards N, Glasheen WP, Villanueva FS, Abbott RD, Kaul S. In vivo myocardial kinetics of air-filled albumin microbubbles during myocardial contrast echocardiography. Comparison with radiolabeled red blood cells. Circ Res. 1994; 74:1157–1165. PMID: 8187282.17. Wei K, Skyba DM, Firschke C, Jayaweera AR, Lindner JR, Kaul S. Interactions between microbubbles and ultrasound: in vitro and in vivo observations. J Am Coll Cardiol. 1997; 29:1081–1088. PMID: 9120163.18. Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a continuous infusion. Circulation. 1998; 97:473–483. PMID: 9490243.19. Le DE, Jayaweera AR, Wei K, Coggins MP, Lindner JR, Kaul S. Changes in myocardial blood volume over a wide range of coronary driving pressures: role of capillaries beyond the autoregulation range. Heart. 2004; 90:1199–1205. PMID: 15367524.20. Lindner JR, Song J, Jayaweera AR, Sklenar J, Kaul S. Microvascular rheology of definity microbubbles after intra-arterial and intravenous administration. J Am Soc Echocardiogr. 2002; 15:396–403. PMID: 12019422.21. Coggins MP, Sklenar J, Le DE, Wei K, Lindner JR, Kaul S. Noninvasive prediction of ultimate infarct size at the time of acute coronary occlusion based on the extent and magnitude of collateralderived myocardial blood flow. Circulation. 2001; 104:2471–2477. PMID: 11705827.22. Kerber RE, Marcus ML, Ehrhardt J, Wilson R, Abboud FM. Corrrelation between echocardiographically demonstrated segmental dyskinesis and regional myocardial perfusion. Circulation. 1975; 52:1097–1104. PMID: 1182955.23. Lieberman AN, Weiss JL, Jugdutt BI, et al. Two-dimensional echocardiography and infarct size: relationship of regional wall motion and thinning to the extent of myocardial infarction in the dog. Circulation. 1981; 63:739–746. PMID: 7471327.24. Lima JA, Becker LC, Melin JA, et al. Impaired thickening of nonischemic myocardium during acute regional ischemia in the dog. Circulation. 1985; 71:1048–1059. PMID: 3986975.25. Force T, Kemper A, Perkins L, Gilfoil M, Cohen C, Parisi AF. Overestimation of infarct size by quantitative two-dimensional echocardiography: the role of tethering and of analytic procedures. Circulation. 1986; 73:1360–1368. PMID: 3698262.26. Weyman AE, Franklin TD Jr, Hogan RD, et al. Importance of temporal heterogeneity in assessing the contraction abnormalities associated with acute myocardial ischemia. Circulation. 1984; 70:102–112. PMID: 6723006.27. Leong-Poi H, Coggins MP, Sklenar J, Jayaweera AR, Wang XQ, Kaul S. Role of collateral blood flow in the apparent disparity between the extent of abnormal wall thickening and perfusion defect size during acute myocardial infarction and demand ischemia. J Am Coll Cardiol. 2005; 45:565–572. PMID: 15708705.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessment of collateral flow and myocardial perfusion by myocardial contrast echocardiography after coronary vasodiator during acute coronary occlusion
- Abnormal Myocardial Blood Flow Reserve Observed in Cardiac Amyloidosis
- Myocardial Contrast Echocardiography for the Assessment of Coronary Blood Flow Reserve
- Assessment of myocardial perfusion status through the angiographically visible collaterals in the ischemic heart disease
- Effect of Regional Hypoxia on Myocardial Blood Flow Through Collateral Circulation in Experimental Canine Model