J Korean Ophthalmol Soc.
2016 Oct;57(10):1631-1639. 10.3341/jkos.2016.57.10.1631.
The Analysis of Retinal Nerve Fiber Layer in Amblyopia Using Spectral Domain Optical Coherence Tomography
- Affiliations
-
- 1Department of Ophthalmology, Maryknoll Hospital, Busan, Korea.
- 2Department of Ophthalmology, Gyeongsang National University Changwon Hospital, Changwon, Korea. pearlsj@hanmail.net
- KMID: 2355421
- DOI: http://doi.org/10.3341/jkos.2016.57.10.1631
Abstract
- PURPOSE
To determine whether retinal nerve fiber layer (RNFL) thickness and optic nerve head parameters differ in the amblyopic and normal fellow eyes of hyperopic anisometropic amblyopic patients using spectral domain optical coherence tomography (SD-OCT).
METHODS
This study included 30 patients with hyperopic anisometropic amblyopia; patient eyes were divided into 30 anisometropic amblyopic eyes and 30 normal fellow eyes. RNFL thickness, disc area, rim area, average cup-to-disc ratio, and cup volume were obtained using SD-OCT. Axial length was obtained using the IOL Master®, and the interocular differences between group were analyzed.
RESULTS
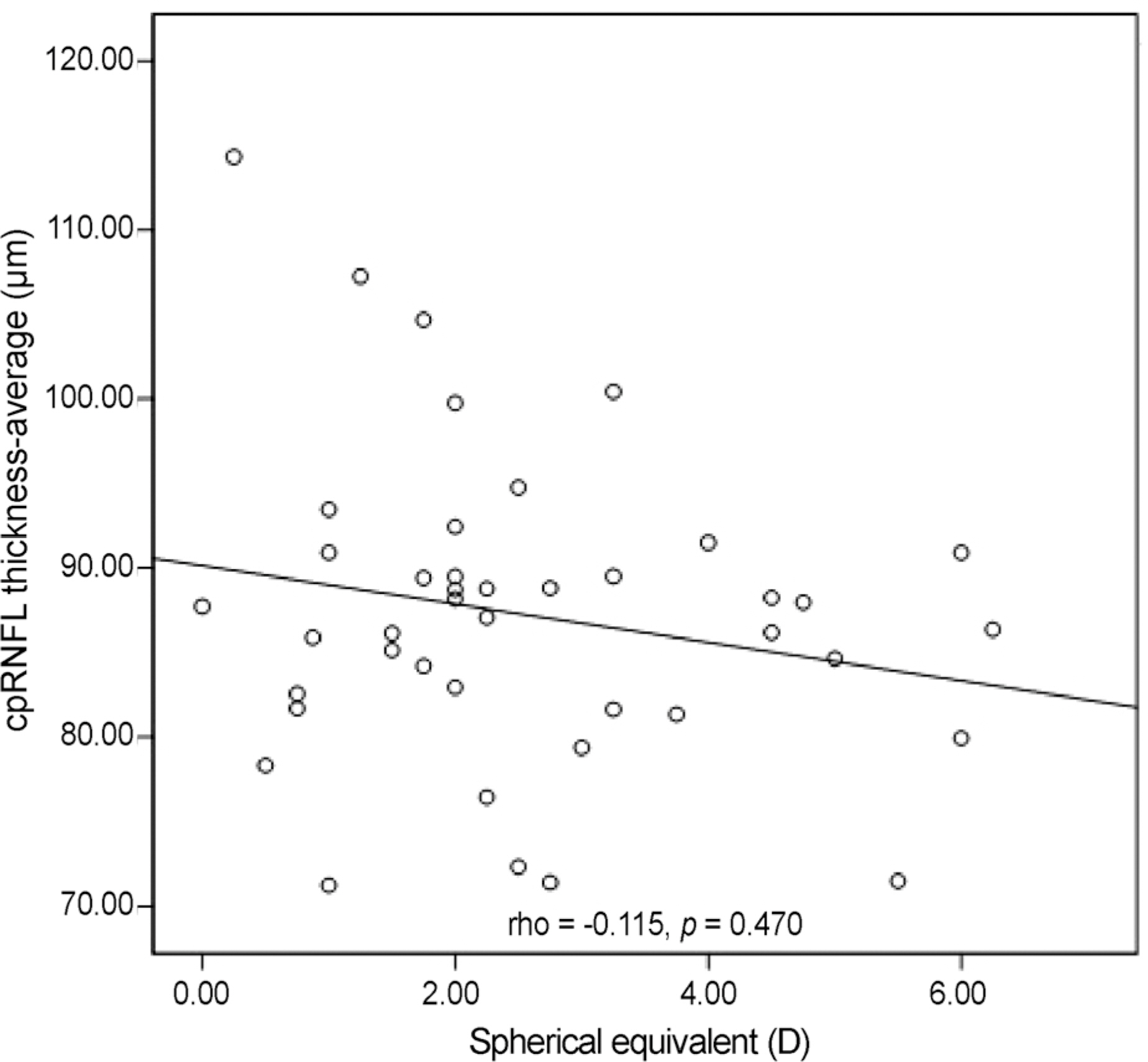
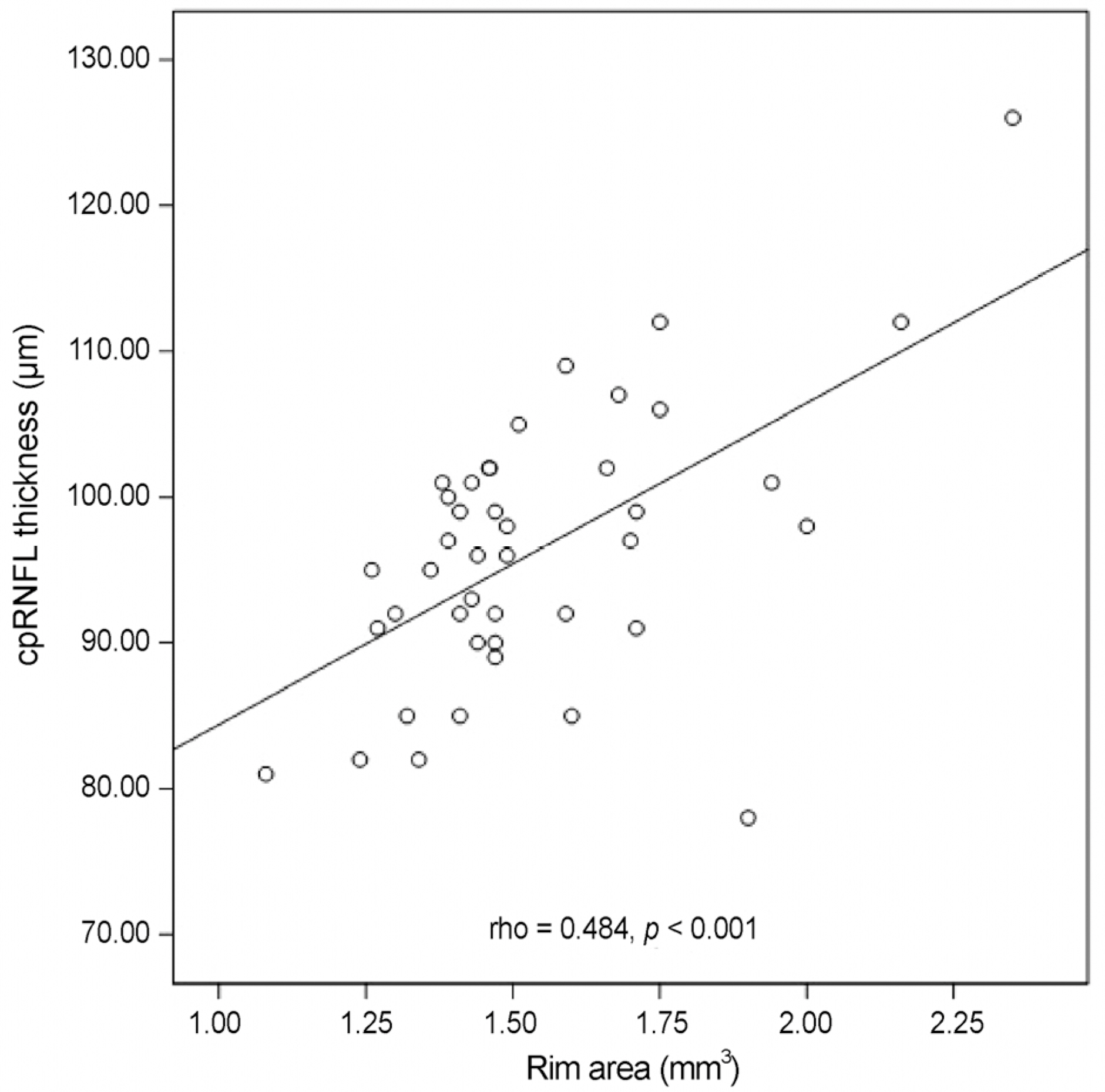
Nasal quadrant RNFL thickness of amblyopic eyes was significantly thicker than that of normal fellow eyes in amblyopic patients (p = 0.010). Among optic nerve parameters, cup volume of amblyopic eyes was significantly smaller than that of normal fellow eyes (p = 0.021). No significant relationship between refractive error and RNFL thickness was observed, and a significant positive linear relationship was observed between neural rim area and RNFL thickness (rho = 0.426, p = 0.005).
CONCLUSIONS
SD-OCT analysis of hyperopic anisometropic amblyopic eyes demonstrated a significant increase in nasal RNFL thickness compared to fellow non-amblyopic eyes. No optic nerve head parameters except cup volume showed significant change.
MeSH Terms
Figure
Reference
-
References
1. Noorden GK. Mechanisms of amblyopia. Adv Ophthalmol. 1977; 34:93–115.2. Von Noorden GK, Campos EC. Amblyopia. Von Noorden GK, Campos EC, editors. Binocular Vision and Ocular Motility. 6th ed.St. Louis: Mosby;2002. chap. 14.3. von Noorden GK. Amblyopia: a multidisciplinary approach. Proctor lecture. Invest Ophthalmol Vis Sci. 1985; 26:1704–16.4. Mcneil NL. Patterns on visual defects in children. Br J Ophthalmol. 1955; 39:688–701.5. Ikeda H, Tremain KE. Amblyopia occurs in retinal ganglion cells in cats reared with convergent squint without alternating fixation. Exp Brain Res. 1979; 35:559–82.
Article6. Von Noorden GK, Crawford ML, Middleditch PR. Effect of lid abdominal on retinal ganglion cells in Macaca mulatta. Brain Res. 1977; 122:437–44.7. Hess RF. Amblyopia: site unseen. Clin Exp Optom. 2001; 84:321–36.
Article8. Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963; 26:1003–17.
Article9. Kiorpes L, Kiper DC, O'Keefe LP, et al. Neuronal correlates of abdominal in the visual cortex of macaque monkeys with experimental strabismus and anisometropia. J Neurosci. 1998; 18:6411–24.10. von Noorden GK. Histological studies of the visual system in monkeys with experimental amblyopia. Invest Ophthalmol. 1973; 12:727–38.11. von Noorden GK, Middleditch PR. Histology of the monkey lateral geniculate nucleus after unilateral lid closure and experimental strabismus: further observations. Invest Ophthalmol. 1975; 14:674–83.12. von Noorden GK, Crawford ML. The lateral geniculate nucleus in human strabismic amblyopia. Invest Ophthalmol Vis Sci. 1992; 33:2729–32.13. Miki A, Liu GT, Goldsmith ZG, et al. Decreased activation of the lateral geniculate nucleus in a patient with anisometropic abdominal demonstrated by functional magnetic resonance imaging. Ophthalmologica. 2003; 217:365–9.14. Hess RF, Thompson B, Gole G, Mullen KT. Deficient responses from the lateral geniculate nucleus in humans with amblyopia. Eur J Neurosci. 2009; 29:1064–70.
Article15. Lempert P, Porter L. Dysversion of the optic disc and axial length measurements in a presumed amblyopic population. J AAPOS. 1998; 2:207–13.
Article16. Lempert P. Optic nerve hypoplasia and small eyes in presumed amblyopia. J AAPOS. 2000; 4:258–66.
Article17. Lempert P. Axial length-disc area ratio in esotropic amblyopia. Arch Ophthalmol. 2003; 121:821–4.
Article18. Lempert P. The axial length/disc area ratio in anisometropic hyperopic amblyopia: a hypothesis for decreased unilateral vision abdominal with hyperopic anisometropia. Ophthalmology. 2004; 111:304–8.19. Lempert P. Retinal area and optic disc rim area in amblyopic, fellow, and normal hyperopic eyes: a hypothesis for decreased acuity in amblyopia. Ophthalmology. 2008; 115:2259–61.
Article20. Yen MY, Cheng CY, Wang AG. Retinal nerve fiber layer thickness in unilateral amblyopia. Invest Ophthalmol Vis Sci. 2004; 45:2224–30.
Article21. Yoon SW, Park WH, Baek SH, Kong SM. Thicknesses of macular retinal layer and peripapillary retinal nerve fiber layer in patients with hyperopic anisometropic amblyopia. Korean J Ophthalmol. 2005; 19:62–7.
Article22. Quoc EB, Delepine B, Tran TH. Thickness of retinal nerve fiber layer and macular volume in children and adults with strabismic and anisometropic amblyopia. J Fr Ophtalmol. 2009; 32:488–95.23. Altintas O, Yüksel N, Ozkan B, Caglar Y. Thickness of the retinal nerve fiber layer, macular thickness, and macular volume in abdominals with strabismic amblyopia. J Pediatr Ophthalmol Strabismus. 2005; 42:216–21.24. Repka MX, Goldenberg-Cohen N, Edwards AR. Retinal nerve fiber layer thickness in amblyopic eyes. Am J Ophthalmol. 2006; 142:247–51.
Article25. Kee SY, Lee SY, Lee YC. Thicknesses of the fovea and retinal nerve fiber layer in amblyopic and normal eyes in children. Korean J Ophthalmol. 2006; 20:177–81.
Article26. Huynh SC, Samarawickrama C, Wang XY, et al. Macular and nerve fiber layer thickness in amblyopia: the Sydney Childhood Eye Study. Ophthalmology. 2009; 116:1604–9.27. Andalib D, Javadzadeh A, Nabai R, Amizadeh Y. Macular and abdominall nerve fiber layer thickness in unilateral anisometropic or strabismic amblyopia. J Pediatr Ophthalmol Strabisums. 2013; 50:218–21.28. Ersan I, Zengin N, Bozkurt B, Ozkagnici A. Evaluation of retinal nerve fiber layer thickness in patients with anisometropic and strabismic amblyopia using optical coherence tomography. J Pediatr Ophthalmol Strabismus. 2013; 50:113–7.
Article29. Wu SQ, Zhu LW, Xu QB, et al. Macular and peripapillary retinal nerve fiber layer thickness in children with hyperopic abdominal amblyopia. Int J Ophthalmol. 2013; 6:85–9.30. Araki S, Miki A, Yamashita T, et al. A comparison between amblyopic and fellow eyes in unilateral amblyopia using spectral-abdominal optical coherence tomography. Clin Ophthalmol. 2014; 8:2199–207.31. Firat PG, Ozsoy E, Demirel S, et al. Evaluation of peripapillary abdominal nerve fiber layer, macula and ganglion cell thickness in abdominal using spectral optical coherence tomography. Int J Ophthalmol. 2013; 6:90–4.32. Al-Haddad CE, Mollayess GM, Cherfan CG, et al. Retinal nerve fibre layer and macular thickness in amblyopia as measured by abdominal-domain optical coherence tomography. Br J Ophthalmol. 2011; 95:1696–9.33. Dickmann A, Petroni S, Perrotta V, et al. Measurement of retinal nerve fiber layer thickness, macular thickness, and foveal volume in amblyopic eyes using spectraldomain optical coherence tomography. J AAPOS. 2012; 16:86–8.
Article34. Park JM, Choi YJ, Kim DH. The analysis of peripapillary RNFL, macula and macular ganglion cell layer thickness in patients with monocular amblyopia using SD-OCT. J Korean Ophthalmol Soc. 2016; 57:98–105.
Article35. Kim YW, Kim SJ, Yu YS. Spectral-domain optical coherence abdominal analysis in deprivational amblyopia: a pilot study with unilateral pediatric cataract patients. Graefes Arch Clin Exp Ophthalmol. 2013; 251:2811–9.36. Aykut V, Öner V, Taş M, et al. Influence of axial length on abdominal retinal nerve fiber layer thickness in children: a study by RTVue spectraldomain optical coherence tomography. Curr Eye Res. 2013; 38:1241–7.37. Öner V, Özgür G, Türkyilmaz K, et al. Effect of axial length on abdominall nerve fiber layer thickness in children. Eur J Ophthalmol. 2014; 24:265–72.38. Littmann H. Determination of the real size of an object on the fundus of the living eye. Klin Monbl Augenheilkd. 1982; 180:286–9.39. Bennett AG, Rudnicka AR, Edgar DF. Improvements on Littmann's method of determining the size of retinal features by fundus photography. Graefes Arch Clin Exp Ophthalmol. 1994; 232:361–7.
Article40. Leung CK, Mohamed S, Leung KS, et al. Retinal nerve fiber layer measurements in myopia: An optical coherence tomography study. Invest Ophthalmol Vis Sci. 2006; 47:5171–6.
Article41. Taylor K, Powell C, Hatt SR, Stewart C. Interventions for uniabdominal and bilateral refractive amblyopia. Cochrane Database Syst Rev. 2012; (4):CD005137.42. Carpineto P, Ciancaglini M, Zuppardi E, et al. Reliability of nerve fiber layer thickness measurements using optical coherence abdominal in normal and glaucomatous eyes. Ophthalmology. 2003; 110:190–5.43. Schuman JS, Pedut-Kloizman T, Pakter H, et al. Optical coherence tomography and histologic measurements of nerve fiber layer thickness in normal and glaucomatous monkey eyes. Invest Ophthalmol Vis Sci. 2007; 48:3645–54.
Article44. Blumenthal EZ, Parikh RS, Pe'er J, et al. Retinal nerve fibre layer imaging compared with histological measurements in a human eye. Eye (Lond). 2009; 23:171–5.
Article45. de Boer JF, Cense B, Park BH, et al. Improved signal-to-noise ratio in spectraldomain compared with time-domain optical coherence tomography. Opt Lett. 2003; 28:2067–9.
Article46. Sherman SM. Visual field defects in monocularly and binocularly deprived cats. Brain Res. 1973; 49:24–45.47. Sparks DL, Mays LE, Gurski MR, Hickey TL. Long- and short-term monocular deprivation in the rhesus monkey: effects on visual fields and optokinetic nystagmus. J Neurosci. 1986; 6:1771–80.
Article48. Wilson JR, Lavallee KA, Joosse MV, et al. Visual fields of monabdominally deprived macaque monkeys. Behav Brain Res. 1989; 33:13–22.49. Maurer D, Lewis TL, Brent HP. Peripheral vision and optokinetic nystagmus in children with unilateral congenital cataract. Behav Brain Res. 1983; 10:151–61.
Article50. Bowering ER, Maurer D, Lewis TL, Brent HP. Sensitivity in the nasal and temporal hemifields in children treated for cataract. Invest Ophthalmol Vis Sci. 1993; 34:3501–9.51. Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA. Mapping the visual field to the optic disc in normal tension abdominal eyes. Ophthalmology. 2000; 107:1809–15.52. Kanamori A, Naka M, Nagai-Kusuhara A, et al. Regional relationship between retinal nerve fiber layer thickness and corresponding visual field sensitivity in glaucomatous eyes. Arch Ophthalmol. 2008; 126:1500–6.
Article53. Archer SM. Amblyopia? J AAPOS. 2000; 4:257.
Article54. Tsai CS, Ritch R, Shin DH, et al. Age-related decline of disc rim area in visually normal subjects. Ophthalmology. 1992; 99:29–35.
Article55. Leung CK, Yu M, Weinreb RN, et al. Retinal nerve fiber layer imaging with spectraldomain optical coherence tomography: a prospective analysis of age-related loss. Ophthalmology. 2012; 119:731–7.56. Varma R, Quigley HA, Pease ME. Changes in optic disk abdominal and number of nerve fibers in experimental glaucoma. Am J Ophthalmol. 1992; 114:554–9.57. Yücel YH, Gupta N, Kalichman MW, et al. Relationship of optic disc topography to optic nerve fiber number in glaucoma. Arch Ophthalmol. 1998; 116:493–7.
Article58. Kanamori A, Kusuhara A, Tatsumi Y, et al. Correlations among GDx-variable corneal compension, optical coherence tomography, and Heidelberg retina tomograph and relationships between these structural parameters and visual field indices. Nippon Ganka Gakkai Zasshi. 2006; 110:180–7.59. Lee J, Kim NR, Kim H, et al. Negative refraction power causes abdominalestimation of peripapillary retinal nerve fibre layer thickness in spectraldomain optical coherence tomography. Br J Ophthalmol. 2011; 95:1284–9.60. Kang SH, Hong SW, Im SK, et al. Effect of myopia on the abdominal of the retinal nerve fiber layer measured by Cirrus HD optical coherence tomography. Invest Ophthalmol Vis Sci. 2010; 51:4075–83.61. Wang XY, Huynh SC, Burlutsky G, et al. Reproducibility of and abdominal of magnification on optical coherence tomography abdominals in children. Am J Ophthalmol. 2007; 143:484–8.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Retinal Nerve Fiber Layer Thickness Measured by Spectral Domain Optical Coherence Tomography in Healthy Koreans
- Spectral-Domain Optical Coherence Tomography Findings in Acute Central Retinal Artery Occlusion
- Fundus Autofluorescence, Fluorescein Angiography and Spectral Domain Optical Coherence Tomography Findings of Retinal Astrocytic Hamartomas in Tuberous Sclerosis
- Thicknesses of the Fovea and Retinal Nerve Fiber Layer in Amblyopic and Normal Eyes in Children
- Thicknesses of Macular Retinal Layer and Peripapillary Retinal Nerve Fiber Layer in Patients with Hyperopic Anisometropic Amblyopia