Korean J Ophthalmol.
2016 Oct;30(5):344-351. 10.3341/kjo.2016.30.5.344.
Anterior Diabetic Retinopathy Studied by Ultra-widefield Angiography
- Affiliations
-
- 1Department of Ophthalmology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. swkang@skku.edu
- 2Department of Ophthalmology, SMG-SNU Boramae Medical Center, Seoul, Korea.
- 3Division of Endocrinology and Metabolism, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 2353827
- DOI: http://doi.org/10.3341/kjo.2016.30.5.344
Abstract
- PURPOSE
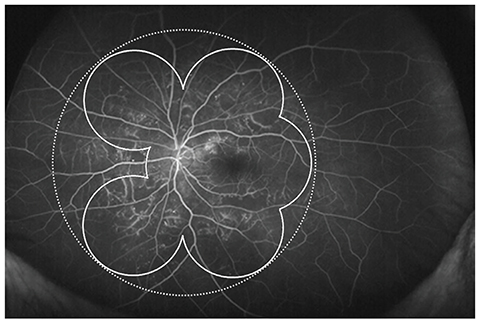
To evaluate the prevalence of anterior type diabetic retinopathy (DR) using ultra-widefield fluorescein angiography and to identify the factors associated with anterior type DR incidence.
METHODS
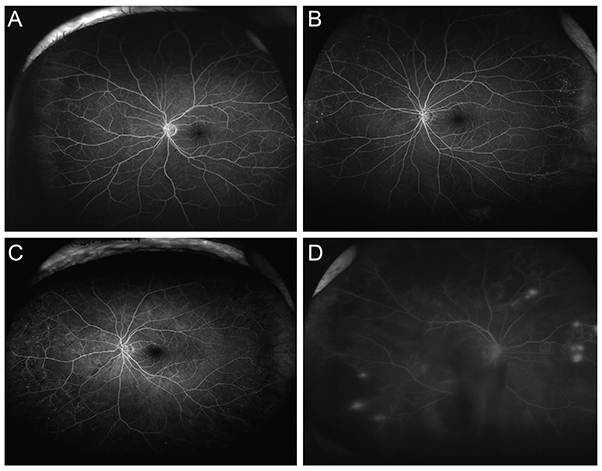
A retrospective case review was used in this study. Patients with non-proliferative diabetic retinopathy (NPDR) underwent examination by ultra-widefield fluorescein angiography, and were classified into anterior, posterior, or diffuse DR groups. Anterior DR was defined if diabetic retinal changes were noted only at the location anterior to the imaginary circle bordered by the Early Treatment Diabetic Retinopathy Study seven-standard fields. Correlations between demographic data, as well as systemic and ocular factors, and the incidence of NPDR types were evaluated.
RESULTS
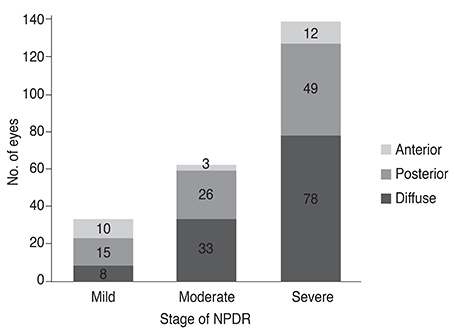
Among the 234 eyes of 234 patients with NPDR, 25 eyes (10.7%) demonstrated anterior DR. Anterior DR was observed in 10 eyes (30.3%) of patients having mild NPDR, three eyes (4.8%) of moderate NPDR patients, and in 12 eyes (7.1%) of severe NPDR patients (p < 0.001). The incidence of anterior DR positively correlated with lower hemoglobin A1c levels and with greater high-density lipoprotein levels following multiple logistic regression analysis (p < 0.001). The mean hemoglobin A1c level was 7.03 ± 0.99% in anterior DR, 7.99 ± 1.74% in posterior DR, and 7.94 ± 1.39% in diffuse DR patients (p = 0.003). The mean high-density lipoprotein level was 51.2 ± 12.5 mg/dL in anterior, 49.7 ± 15.2 mg/dL in posterior, and 45.2 ± 13.1 mg/dL in diffuse DR patients (p = 0.010).
CONCLUSIONS
Diabetic retinal changes confined to an anterior location were more frequently noted in earlier stages of NPDR. The incidence of DR sparing posterior retinal involvement was related to favorable blood sugar and lipid profiles.
MeSH Terms
Figure
Reference
-
1. Fundus photographic risk factors for progression of diabetic retinopathy: ETDRS report number 12. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991; 98:5 Suppl. 823–833.2. Manivannan A, Plskova J, Farrow A, et al. Ultra-wide-field fluorescein angiography of the ocular fundus. Am J Ophthalmol. 2005; 140:525–527.3. Patel RD, Messner LV, Teitelbaum B, et al. Characterization of ischemic index using ultra-widefield fluorescein angiography in patients with focal and diffuse recalcitrant diabetic macular edema. Am J Ophthalmol. 2013; 155:1038–1044.e2.4. Wessel MM, Aaker GD, Parlitsis G, et al. Ultra-wide-field angiography improves the detection and classification of diabetic retinopathy. Retina. 2012; 32:785–791.5. Ruilope LM, Segura J. Losartan and other angiotensin II antagonists for nephropathy in type 2 diabetes mellitus: a review of the clinical trial evidence. Clin Ther. 2003; 25:3044–3064.6. Malaguarnera G, Gagliano C, Bucolo C, et al. Lipoprotein(a) serum levels in diabetic patients with retinopathy. Biomed Res Int. 2013; 2013:943505.7. Lyons TJ, Jenkins AJ, Zheng D, et al. Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci. 2004; 45:910–918.8. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus: the Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993; 329:977–986.9. The absence of a glycemic threshold for the development of long-term complications: the perspective of the Diabetes Control and Complications Trial. Diabetes. 1996; 45:1289–1298.10. The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy in the diabetes control and complications trial. Diabetes. 1995; 44:968–983.11. Klein R, Klein BE, Moss SE. Relation of glycemic control to diabetic microvascular complications in diabetes mellitus. Ann Intern Med. 1996; 124(1 Pt 2):90–96.12. Toth PP, Simko RJ, Palli SR, et al. The impact of serum lipids on risk for microangiopathy in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2012; 11:109.13. ACCORD Study Group. ACCORD Eye Study Group. Chew EY, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010; 363:233–244.14. Knowler WC, Bennett PH, Ballintine EJ. Increased incidence of retinopathy in diabetics with elevated blood pressure: a six-year follow-up study in Pima Indians. N Engl J Med. 1980; 302:645–650.15. Rand LI, Prud'homme GJ, Ederer F, Canner PL. Factors influencing the development of visual loss in advanced diabetic retinopathy: Diabetic Retinopathy Study (DRS) report No. 10. Invest Ophthalmol Vis Sci. 1985; 26:983–991.16. Janka HU, Warram JH, Rand LI, Krolewski AS. Risk factors for progression of background retinopathy in long-standing IDDM. Diabetes. 1989; 38:460–464.17. Chew EY, Klein ML, Ferris FL 3rd, et al. Association of elevated serum lipid levels with retinal hard exudate in diabetic retinopathy: Early Treatment Diabetic Retinopathy Study (ETDRS) Report 22. Arch Ophthalmol. 1996; 114:1079–1084.18. Kaneko Y, Moriyama M, Hirahara S, et al. Areas of nonperfusion in peripheral retina of eyes with pathologic myopia detected by ultra-widefield fluorescein angiography. Invest Ophthalmol Vis Sci. 2014; 55:1432–1439.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Simplified Correction of Ischemic Index in Diabetic Retinopathy Evaluated by Ultra-widefield Fluorescein Angiography
- Clinical Analysis of Retinopathy of Juvenile Diabetes
- The Influences of Arteriosclerosis on the Development and Progression of Diabetic Retinopathy
- Clinical Analysis of Diabetic Retinopathy According to the Type of Diabetes Mellitus
- Photopic Flash ERG Changes in Diabetic Retinopathy: with reference to severity of diabetic retinopathy