J Pathol Transl Med.
2016 Sep;50(5):361-368. 10.4132/jptm.2016.06.19.
CD99 Is Strongly Expressed in Basal Cells of the Normal Adult Epidermis and Some Subpopulations of Appendages: Comparison with Developing Fetal Skin
- Affiliations
-
- 1Graduate School of Medicine, University of Ulsan College of Medicine, Seoul, Korea.
- 2Mizmedi Hospital, Seoul, Korea.
- 3Department of Pathology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea. csikpark@amc.seoul.kr
- KMID: 2353598
- DOI: http://doi.org/10.4132/jptm.2016.06.19
Abstract
- BACKGROUND
CD99 is a cell surface transmembrane glycoprotein expressed in various tissues. CD99 is differentially expressed between subpopulations of each tissue and is highly expressed in certain hematopoietic and precursor cells. However, there has been no comprehensive study of CD99 expression in normal skin. We evaluated CD99 expression in normal human skin and developing fetal skin.
METHODS
Seventy-five adult skin samples containing normal skin and eight fetal skin samples of different gestational ages were collected. CD99 immunohistochemical staining was performed to evaluate expression pattern in adult and fetal skin samples. CD99 and CD34 expression were compared by double immunofluorescence.
RESULTS
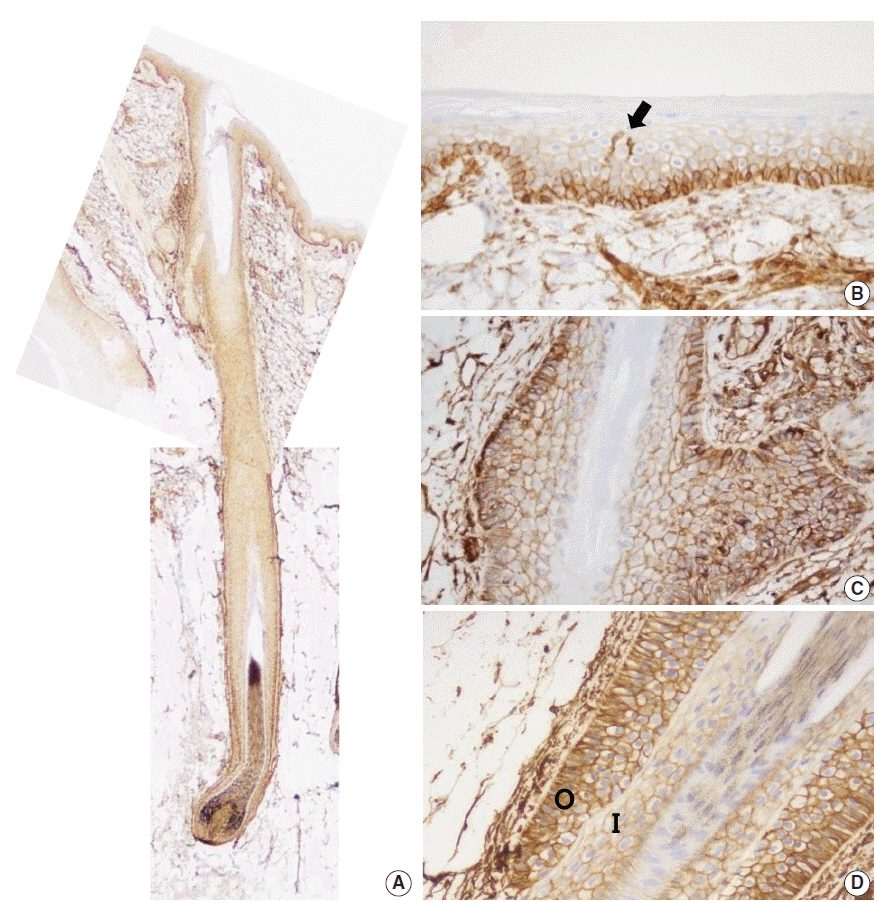
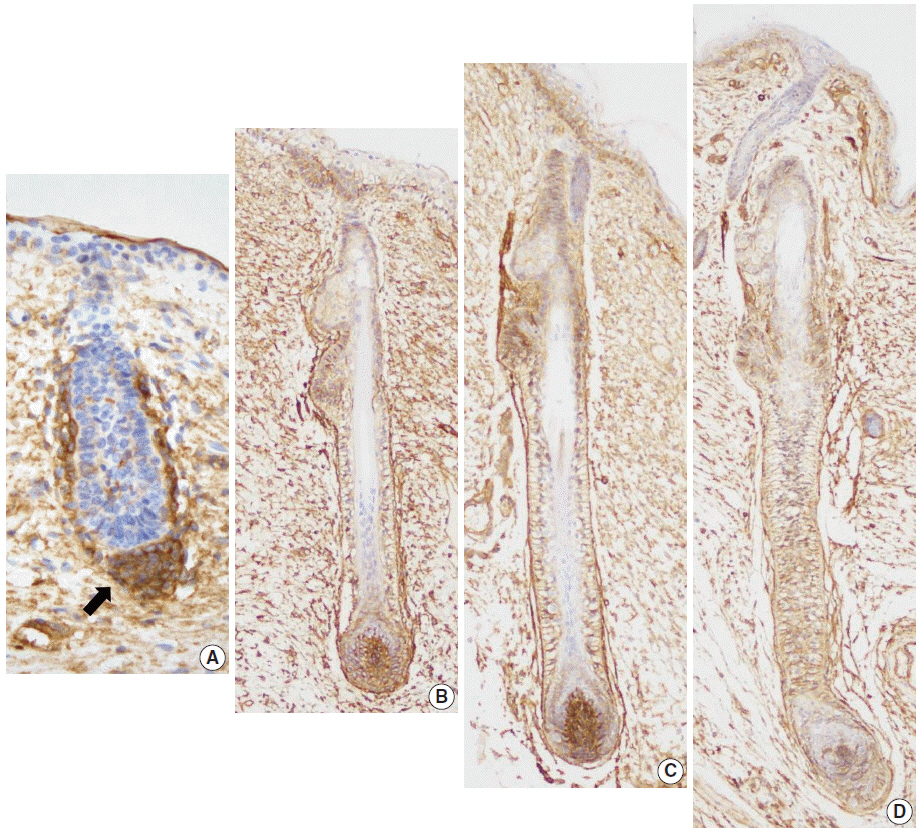
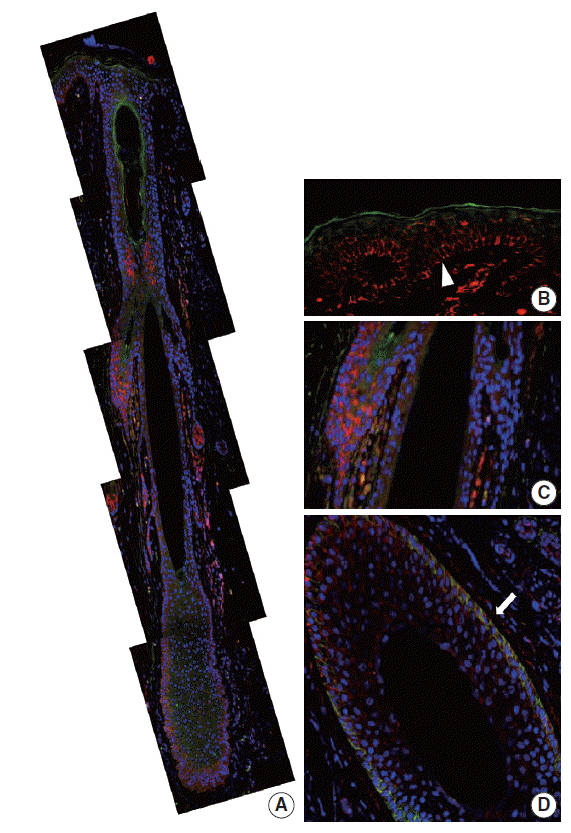
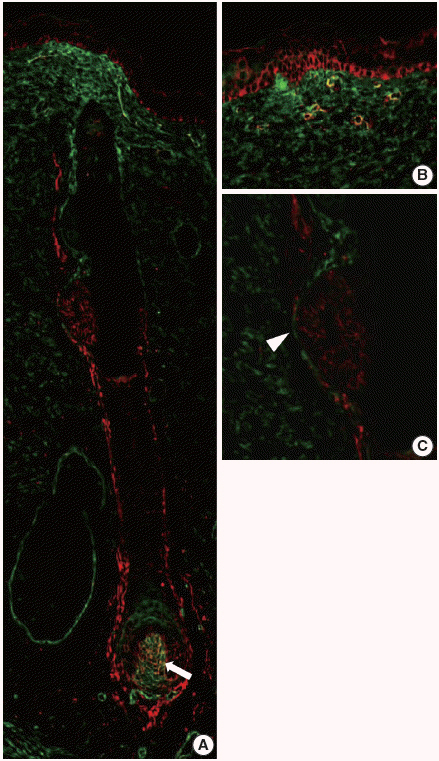
In normal adult skin, CD99 was strongly expressed in the membrane of epidermal basal keratinocytes, hair follicle bulges and outer root sheaths, and inner secretory cells of eccrine sweat glands. In fetal skin, CD99 was not expressed on the periderm at 16 weeks of gestation but was expressed in basal cells of fetal skin at around 19 weeks of gestation. CD99 expression became comparable to that of the adult skin after 20 weeks of gestation. CD99 and CD34 were co-expressed in hair follicle outer root sheaths, as seen by double immunofluorescence study.
CONCLUSIONS
This is the first study examining CD99 expression pattern in normal adult and fetal skin. CD99 tends to be expressed in the basal/precursor cells of epidermis and in hair follicles. These results provide a basis for future investigation on functions of CD99 in the skin and provide a novel potential target for the treatment of dermatologic lesions.
Keyword
MeSH Terms
Figure
Reference
-
1. Aussel C, Bernard G, Breittmayer JP, Pelassy C, Zoccola D, Bernard A. Monoclonal antibodies directed against the E2 protein (MIC2 gene product) induce exposure of phosphatidylserine at the thymocyte cell surface. Biochemistry. 1993; 32:10096–101.
Article2. Dworzak MN, Fritsch G, Buchinger P, et al. Flow cytometric assessment of human MIC2 expression in bone marrow, thymus, and peripheral blood. Blood. 1994; 83:415–25.
Article3. Gordon MD, Corless C, Renshaw AA, Beckstead J. CD99, keratin, and vimentin staining of sex cord-stromal tumors, normal ovary, and testis. Mod Pathol. 1998; 11:769–73.4. Strauchen JA, Miller LK. Lymphoid progenitor cells in human tonsils. Int J Surg Pathol. 2003; 11:21–4.
Article5. Bernard G, Zoccola D, Deckert M, Breittmayer JP, Aussel C, Bernard A. The E2 molecule (CD99) specifically triggers homotypic aggregation of CD4+ CD8+ thymocytes. J Immunol. 1995; 154:26–32.6. Pettersen RD, Bernard G, Olafsen MK, Pourtein M, Lie SO. CD99 signals caspase-independent T cell death. J Immunol. 2001; 166:4931–42.
Article7. Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002; 3:143–50.
Article8. Hahn JH, Kim MK, Choi EY, et al. CD99 (MIC2) regulates the LFA-1/ICAM-1-mediated adhesion of lymphocytes, and its gene encodes both positive and negative regulators of cellular adhesion. J Immunol. 1997; 159:2250–8.9. Kim SH, Choi EY, Shin YK, et al. Generation of cells with Hodgkin’s and Reed-Sternberg phenotype through downregulation of CD99 (Mic2). Blood. 1998; 92:4287–95.
Article10. Bixel G, Kloep S, Butz S, Petri B, Engelhardt B, Vestweber D. Mouse CD99 participates in T-cell recruitment into inflamed skin. Blood. 2004; 104:3205–13.
Article11. Ambros IM, Ambros PF, Strehl S, Kovar H, Gadner H, Salzer-Kuntschik M. MIC2 is a specific marker for Ewing’s sarcoma and peripheral primitive neuroectodermal tumors: evidence for a common histogenesis of Ewing’s sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer. 1991; 67:1886–93.
Article12. Narisawa Y, Kohda H. Two- and three-dimensional demonstrations of morphological alterations of early anagen hair follicle with special reference to the bulge area. Arch Dermatol Res. 1996; 288:98–102.
Article13. Young B, Lowe JS, Stevens A, Heath JW. Wheater’s functional histology: a text and colour atlas. 5th ed. Philadelphia: Elsevier;2006. p. 167–85.14. Park CK, Shin YK, Kim TJ, Park SH, Ahn GH. High CD99 expression in memory T and B cells in reactive lymph nodes. J Korean Med Sci. 1999; 14:600–6.
Article15. Cho EY, Choi Y, Chae SW, Sohn JH, Ahn GH. Immunohistochemical study of the expression of adhesion molecules in ovarian serous neoplasms. Pathol Int. 2006; 56:62–70.
Article16. Shin SJ, Lee H, Jung G, et al. Expression of CD99 in multiple myeloma: a clinicopathologic and immunohistochemical study of 170 cases. Korean J Pathol. 2014; 48:209–16.
Article17. Holbrook KA. Structure and function of the developing human skin. In : Goldsmith LA, editor. Biochemistry and physiology of the skin. New York: Oxford University Press;1983. p. 64–101.18. Wilkerson AE, Glasgow MA, Hiatt KM. Immunoreactivity of CD99 in invasive malignant melanoma. J Cutan Pathol. 2006; 33:663–6.
Article19. Liu HW, Cheng B, Li JF, et al. Characterization of angiotensin-converting enzyme expression during epidermis morphogenesis in humans: a potential marker for epidermal stem cells. Br J Dermatol. 2009; 160:250–8.
Article20. Jiang S, Zhao L, Purandare B, Hantash BM. Differential expression of stem cell markers in human follicular bulge and interfollicular epidermal compartments. Histochem Cell Biol. 2010; 133:455–65.
Article21. Inoue K, Aoi N, Sato T, et al. Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. Lab Invest. 2009; 89:844–56.
Article22. Hoang MP, Keady M, Mahalingam M. Stem cell markers (cytokeratin 15, CD34 and nestin) in primary scarring and nonscarring alopecia. Br J Dermatol. 2009; 160:609–15.
Article23. Gho CG, Braun JE, Tilli CM, Neumann HA, Ramaekers FC. Human follicular stem cells: their presence in plucked hair and follicular cell culture. Br J Dermatol. 2004; 150:860–8.
Article24. Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci U S A. 2001; 98:3156–61.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of Low Molecular Weight Keratin (K8/18) in Fetal Skin Development
- Significance of CD99 Immunoreactive Cells in relation to Gastrin-producing Cells in Human Gastric Mucosa
- Immunohistochemical Study on the Expression of Desmocollin 1 during Skin Development
- Immunohistochemical Expression of the alpha- and gamma-Catenin in the Fetal Skin Development
- Immunohistochemical Study on Cytokeratin Expression in Epidermis of Human Fetus