J Korean Assoc Oral Maxillofac Surg.
2016 Apr;42(2):90-98. 10.5125/jkaoms.2016.42.2.90.
Demineralized dentin matrix combined with recombinant human bone morphogenetic protein-2 in rabbit calvarial defects
- Affiliations
-
- 1R&D Institute, Korea Tooth Bank, Seoul, Korea.
- 2Department of Medicine, Korea University Graduate School, Seoul, Korea.
- 3Department of Oral and Maxillofacial Surgery, Section of Dentistry, Seoul National University Bundang Hospital, Seongnam, Korea.
- 4Department of Oral and Maxillofacial Surgery, College of Dentistry, Dankook University, Cheonan, Korea.
- 5Department of Dentistry, Korea University Anam Hospital, Seoul, Korea. koprosth@unitel.co.kr
- 6Department of Dentistry, Korea University Ansan Hospital, Ansan, Korea. omfs1109@korea.ac.kr
- KMID: 2351731
- DOI: http://doi.org/10.5125/jkaoms.2016.42.2.90
Abstract
OBJECTIVES
The aim of this study was to compare the osteogenic effects of demineralized dentin matrix (DDM) combined with recombinant human bone morphogenetic protein-2 (rhBMP-2) in rabbit calvarial defects with DDM and anorganic bovine bone (ABB) combined with rhBMP-2.
MATERIALS AND METHODS
Four round defects with 8-mm diameters were created in each rabbit calvaria. Each defect was treated with one of the following: 1) DDM, 2) ABB/rhBMP-2, or 3) DDM/rhBMP-2. The rhBMP-2 was combined with DDM and ABB according to a stepwise dry and dip lyophilizing protocol. Histological and microcomputed tomography (µCT) analyses were performed to measure the amount of bone formation and bone volume after 2- and 8-week healing intervals.
RESULTS
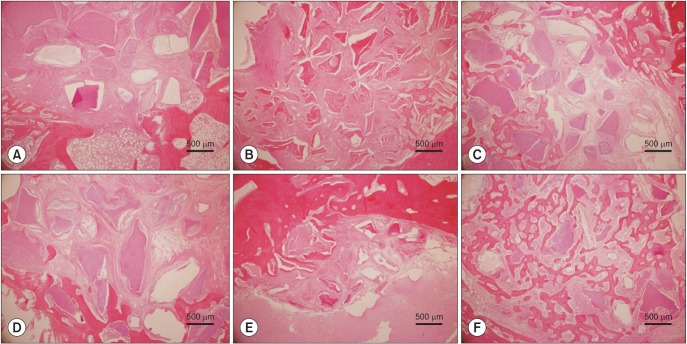
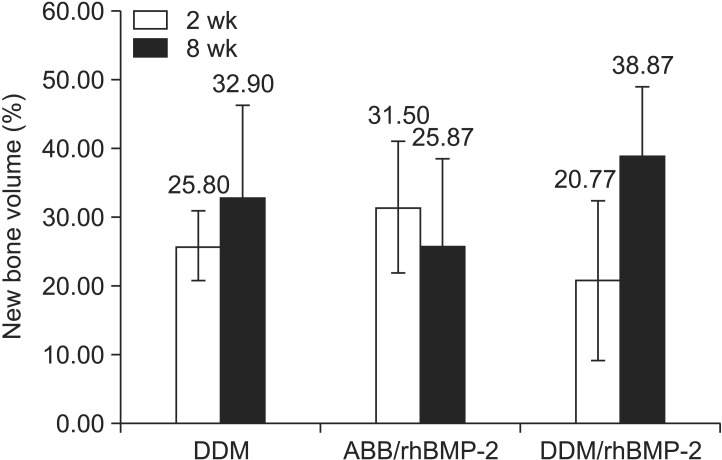
Upon histological observation at two weeks, the DDM and ABB/rhBMP-2 groups showed osteoconductive bone formation, while the DDM/rhBMP-2 group showed osteoconductive and osteoinductive bone formation. New bone formation was higher in DDM/rhBMP-2, DDM and ABB decreasing order. The amounts of bone formation were very similar at two weeks; however, at eight weeks, the DDM/rhBMP-2 group showed a two-fold greater amount of bone formation compared to the DDM and ABB/rhBMP-2 groups. The µCT analysis showed markedly increased bone volume in the DDM/rhBMP-2 group at eight weeks compared with that of the DDM group. Notably, there was a slight decrease in bone volume in the ABB/rhBMP-2 group at eight weeks. There were no significant differences among the DDM, ABB/rhBMP-2, and DDM/rhBMP-2 groups at two or eight weeks.
CONCLUSION
Within the limitations of this study, DDM appears to be a suitable carrier for rhBMP-2 in orthotopic sites.
Keyword
Figure
Reference
-
1. Govender S, Csimma C, Genant HK, Valentin-Opran A, Amit Y, Arbel R, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002; 84:2123–2134. PMID: 12473698.2. Burkus JK, Heim SE, Gornet MF, Zdeblick TA. Is INFUSE bone graft superior to autograft bone? An integrated analysis of clinical trials using the LT-Cage lumbar tapered fusion device. J Spinal Disord Tech. 2003; 16:113–122. PMID: 12679664.
Article3. Ripamonti U, Reddi AH. Periodontal regeneration: potential role of bone morphogenetic proteins. J Periodontal Res. 1994; 29:225–235. PMID: 7932015.
Article4. Asahina I. Bone morphogenetic proteins: their history and characteristics. J Hard Tissue Biol. 2014; 23:283–286.
Article5. Gauthier O, Bouler JM, Aguado E, Pilet P, Daculsi G, Macroporous . Biphasic calcium phosphate ceramics: influence of macropore diameter and macroporosity percentage on bone ingrowth. Biomaterials. 1998; 19:133–139. PMID: 9678860.6. Kim CS, Kim JI, Kim J, Choi SH, Chai JK, Kim CK, et al. Ectopic bone formation associated with recombinant human bone morphogenetic proteins-2 using absorbable collagen sponge and beta tricalcium phosphate as carriers. Biomaterials. 2005; 26:2501–2507. PMID: 15585252.
Article7. Hyun SJ, Han DK, Choi SH, Chai JK, Cho KS, Kim CK, et al. Effect of recombinant human bone morphogenetic protein-2, -4, and -7 on bone formation in rat calvarial defects. J Periodontol. 2005; 76:1667–1674. PMID: 16253088.
Article8. Triplett RG, Nevins M, Marx RE, Spagnoli DB, Oates TW, Moy PK, et al. Pivotal randomized, parallel evaluation of recombinant human bone morphogenetic protein-2/absorbable collagen sponge and autogenous bone graft for maxillary sinus floor augmentation. J Oral Maxillofac Surg. 2009; 67:1947–1960. PMID: 19686934.
Article9. Ike M, Urist MR. Recycled dentin root matrix for a carrier of recombinant human bone morphogenetic protein. J Oral Implantol. 1998; 24:124–132. PMID: 9893518.
Article10. Murata M. Bone engineering using human demineralixed dentin matrix and recombinant human BMP-2. J Hard Tissue Biol. 2005; 14:80–81.11. Kim YK, Um IW, An HJ, Kim KW, Hong KS, Murata M. Effects of demineralized dentin matrix used as an rhBMP-2 carrier for bone regeneration. J Hard Tissue Biol. 2014; 23:415–422.
Article12. Kim YK, Kwon KH, Lee ES, Kim CH, Kim MY, Um IW. Experimental study on human demineralized dentin matrix as rhBMP-2 carrier in vivo. J Dent App. 2015; 2:269–273.13. Jung JH, Yun JH, Um YJ, Jung UW, Kim CS, Choi SH, et al. Bone formation of Escherichia coli expressed rhBMP-2 on absorbable collagen block in rat calvarial defects. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2011; 111:298–305. PMID: 20875759.
Article14. Geiger M, Li RH, Friess W. Collagen sponges for bone regeneration with rhBMP-2. Adv Drug Deliv Rev. 2003; 55:1613–1629. PMID: 14623404.
Article15. Fiorellini JP, Howell TH, Cochran D, Malmquist J, Lilly LC, Spagnoli D, et al. Randomized study evaluating recombinant human bone morphogenetic protein-2 for extraction socket augmentation. J Periodontol. 2005; 76:605–613. PMID: 15857102.
Article16. McKay WF, Peckham SM, Marotta JS. The science of rhBMP-2. St. Louis: Quality Medical Publishing;2006.17. Schützenberger S, Schultz A, Hausner T, Hopf R, Zanoni G, Morton T, et al. The optimal carrier for BMP-2: a comparison of collagen versus fibrin matrix. Arch Orthop Trauma Surg. 2012; 132:1363–1370. PMID: 22660797.
Article18. Jung SY, KO YJ, Jang HS, Kang SW, Park JH. The effect of carrier for BMP-2 delivery on histological aspects of tissue-engineered bone. Tissue Eng Regen Med. 2013; 10:341–346.
Article19. Schmitt C, Lutz R, Doering H, Lell M, Ratky J, Schlegel KA. Bio-Oss block combined with BMP-2 and VEGF for the regeneration of bony defects and vertical augmentation. Clin Oral Implants Res. 2013; 24:450–460. PMID: 22092937.20. Kim YK, Um IW, Murata M. Tooth bank system for bone regeneration: safety report. J Hard Tissue Biol. 2014; 23:371–376.21. Kim YK, Kim SG, Byeon JH, Lee HJ, UM IU, Lim SC, et al. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010; 109:496–503. PMID: 20060336.
Article22. Murata M, Kawai T, Kawakami T, Akazawa T, Tazaki J, Ito K, et al. Human acid-insoluble dentin with BMP-2 accelerates bone induction in subcutaneous and intramuscular tissues. J Ceram Soc Jpn. 2010; 118:438–441.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bone regeneration of demineralized dentin matrix with platelet-rich fibrin and recombinant human bone morphogenetic protein-2 on the bone defects in rabbit calvaria
- Bone Induction by Demineralized Dentin Matrix in Nude Mouse Muscles
- Postulated release profile of recombinant human bone morphogenetic protein-2 (rhBMP-2) from demineralized dentin matrix
- Cranial bone regeneration according to different particle sizes and densities of demineralized dentin matrix in the rabbit model
- Effect of bone wax plus bone morphogenetic protein into rabbit calvarial defects