J Korean Assoc Oral Maxillofac Surg.
2019 Jun;45(3):123-128. 10.5125/jkaoms.2019.45.3.123.
Postulated release profile of recombinant human bone morphogenetic protein-2 (rhBMP-2) from demineralized dentin matrix
- Affiliations
-
- 1R&D Institute, Korea Tooth Bank, Seoul, Korea. h-bmp@hanmail.net
- 2Department of Oral and Maxillofacial Surgery, Section of Dentistry, Armed Forces Capital Hospital, Seongnam, Korea. kujk123@gmail.com
- 3Department of Oral and Maxillofacial Surgery, Seoul Asan Medical Center, Seoul, Korea.
- 4Department of Oral and Maxillofacial Surgery, Section of Dentistry, Seoul National University Bundang Hospital, Seongnam, Korea.
- 5Department of Oral and Maxillofacial Surgery, Institute of Oral Health Science, Ajou University School of Medicine, Suwon, Korea.
- 6Department of Dental Implant/Oral Surgery, Private Clinic, Seoul, Korea.
- KMID: 2451318
- DOI: http://doi.org/10.5125/jkaoms.2019.45.3.123
Abstract
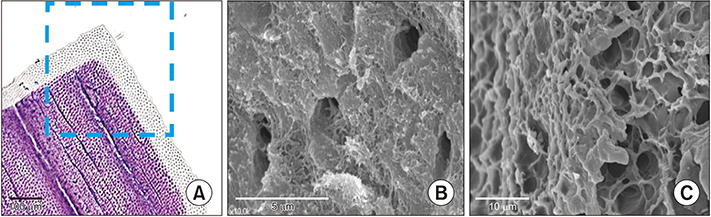
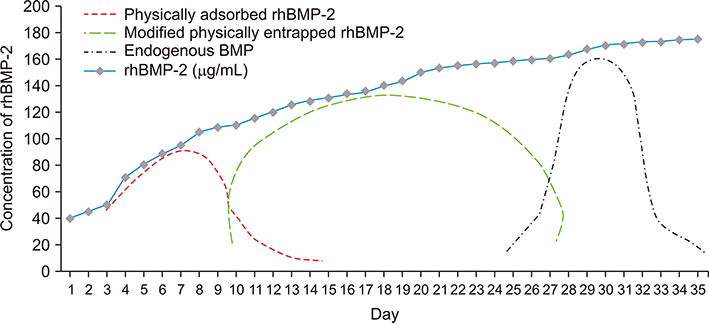
- Demineralized dentin matrix (DDM) has been used as a recombinant human bone morphogenetic protein-2 (rhBMP-2) carrier in many clinical trials. To optimize the clinical safety and efficacy of rhBMP-2 with DDM, efforts have been made to improve the delivery of rhBMP-2 by 1) lowering the administered dose, 2) localizing the protein, and 3) prolonging its retention time at the action site as well as the bone forming capacity of the carrier itself. The release profile of rhBMP-2 that is associated with endogenous BMP in dentin has been postulated according to the type of incorporation, which is attributed to the loosened interfibrillar space and nanoporous dentinal tubule pores. Physically adsorbed and modified, physically entrapped rhBMP-2 is sequentially released from the DDM surface during the early stage of implantation. As DDM degradation progresses, the loosened interfibrillar space and enlarged dentinal tubules release the entrapped rhBMP-2. Finally, the endogenous BMP in dentin is released with osteoclastic dentin resorption. According to the postulated release profile, DDM can therefore be used in a controlled manner as a sequential delivery scaffold for rhBMP-2, thus sustaining the rhBMP-2 concentration for a prolonged period due to localization. In addition, we attempted to determine how to lower the rhBMP-2 concentration to 0.2 mg/mL, which is lower than the approved 1.5 mg/mL.
MeSH Terms
Figure
Reference
-
1. Urist MR. Bone: formation by autoinduction. Science. 1965; 150:893–899.
Article2. Urist MR, Dowell TA, Hay PH, Strates BS. Inductive substrates for bone formation. Clin Orthop Relat Res. 1968; 59:59–96.
Article3. Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, et al. Novel regulators of bone formation: molecular clones and activities. Science. 1988; 242:1528–1534.
Article4. Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998; 16:247–252.
Article5. Boyne PJ, James RA. Grafting of the maxillary sinus floor with autogenous marrow and bone. J Oral Surg. 1980; 38:613–616.6. Moslemi N, Khoshkam V, Rafiei SC, Bahrami N, Aslroosta H. Outcomes of alveolar ridge preservation with recombinant human bone morphogenetic protein-2: a systematic review. Implant Dent. 2018; 27:351–362.
Article7. Haidar ZS, Hamdy RC, Tabrizian M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol Lett. 2009; 31:1825–1835.
Article8. Boyne PJ. Animal studies of application of rhBMP-2 in maxillofacial reconstruction. Bone. 1996; 19:1 Suppl. 83S–92S.
Article9. Lee SH, Shin H. Matrices and scaffolds for delivery of bioactive molecules in bone and cartilage tissue engineering. Adv Drug Deliv Rev. 2007; 59:339–359.
Article10. Lo KW, Ulery BD, Ashe KM, Laurencin CT. Studies of bone morphogenetic protein-based surgical repair. Adv Drug Deliv Rev. 2012; 64:1277–1291.
Article11. James AW, LaChaud G, Shen J, Asatrian G, Nguyen V, Zhang X, et al. A review of the clinical side effects of bone morphogenetic protein-2. Tissue Eng Part B Rev. 2016; 22:284–297.
Article12. U.S. Food and Drug Administration. Summary of safety and effectiveness data [Internet]. Silver Spring (MD): U.S. Food and Drug Administration;2007. 03. 09. cited 2018 Dec 30. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf5/P050053b.pdf.13. Ike M, Urist MR. Recycled dentin root matrix for a carrier of recombinant human bone morphogenetic protein. J Oral Implantol. 1998; 24:124–132.
Article14. Murata M, Kawai T, Kawakami T, Akazawa T, Tazaki J, Ito K, et al. Human acid-insoluble dentin with BMP-2 accelerates bone induction in subcutaneous and intramuscular tissues. J Ceram Soc Jpn. 2010; 118:438–441.
Article15. Miyaji H, Kawanami M. Dentin conditioning with BMP for reconstruction of periodontal attachment. In : Murata M, Um IW, editors. Advances in oral tissue engineering. Chicago: Quintessence Publishing;2014. p. 19–24.16. Kim YK, Um IW, An HJ, Kim KW, Hong KS, Murata M. Effects of demineralized dentin matrix used as an rhBMP-2 carrier for bone regeneration. J Hard Tissue Biol. 2014; 23:415–422.
Article17. Um IW, Kim YK, Jun SH, Kim MY, Cui N. Demineralized dentin matrix as a carrier of recombinant human bone morphogenetic proteins: in vivo study. J Hard Tissue Biol. 2018; 27:219–226.
Article18. Bessho K, Tanaka N, Matsumoto J, Tagawa T, Murata M. Human dentin-matrix-derived bone morphogenetic protein. J Dent Res. 1991; 70:171–175.
Article19. Murata M. Collagen biology for bone regenerative surgery. J Korean Assoc Oral Maxillofac Surg. 2012; 38:321–325.
Article20. Kim YK, Kim SG, Yun PY, Yeo IS, Jin SC, Oh JS, et al. Autogenous teeth used for bone grafting: a comparison with traditional grafting materials. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014; 117:e39–e45.
Article21. Pang KM, Um IW, Kim YK, Woo JM, Kim SM, Lee JH. Autogenous demineralized dentin matrix from extracted tooth for the augmentation of alveolar bone defect: a prospective randomized clinical trial in comparison with anorganic bovine bone. Clin Oral Implants Res. 2017; 28:809–815.
Article22. Schilke R, Lisson JA, Bauss O, Geurtsen W. Comparison of the number and diameter of dentinal tubules in human and bovine dentine by scanning electron microscopic investigation. Arch Oral Biol. 2000; 45:355–361.
Article23. Coutinho ET, d'Almeida JRM, Paciornik S. Evaluation of microstructural parameters of human dentin by digital image analysis. Mater Res. 2007; 10:153–159.
Article24. Goldberg M, Kulkarni AB, Young M, Boskey A. Dentin: structure, composition and mineralization. Front Biosci (Elite Ed). 2011; 3:711–735.
Article25. Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A. Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res. 2004; 83:590–595.
Article26. Kim SY, Kim YK, Park YH, Park JC, Ku JK, Um IW, et al. Evaluation of the healing potential of demineralized dentin matrix fixed with recombinant human bone morphogenetic protein-2 in bone grafts. Materials (Basel). 2017; 10:E1049.
Article27. Kim YK, Um IW, Murata M. Tooth bank system for bone regeneration: safety report. J Hard Tissue Biol. 2014; 23:371–376.28. Nakabayashi N, Pashley DH. Hybridization of dental hard tissues. Tokyo: Quintessence Publishing;1998.29. Garberoglio R, Brännström M. Scanning electron microscopic investigation of human dentinal tubules. Arch Oral Biol. 1976; 21:355–362.
Article30. Luginbuehl V, Meinel L, Merkle HP, Gander B. Localized delivery of growth factors for bone repair. Eur J Pharm Biopharm. 2004; 58:197–208.
Article31. Um IW, Kim YK, Park JC, Lee JH. Clinical application of autogenous demineralized dentin matrix loaded with recombinant human bone morphogenetic-2 for socket preservation: a case series. Clin Implant Dent Relat Res. 2019; 21:4–10.
Article32. Vennat E, Bogicevic C, Fleureau JM, Degrange M. Demineralized dentin 3D porosity and pore size distribution using mercury porosimetry. Dent Mater. 2009; 25:729–735.
Article33. Olthof MGL, Kempen DHR, Liu X, Dadsetan M, Tryfonidou MA, Yaszemski MJ, et al. Bone morphogenetic protein-2 release profile modulates bone formation in phosphorylated hydrogel. J Tissue Eng Regen Med. 2018; 12:1339–1351.
Article34. Fosse G, Saele PK, Eide R. Numerical density and distributional pattern of dentin tubules. Acta Odontol Scand. 1992; 50:201–210.
Article35. Kim KW. Bone induction by demineralized dentin matrix in nude mouse muscles. Maxillofac Plast Reconstr Surg. 2014; 36:50–56.
Article36. Tanoue R, Ohta K, Miyazono Y, Iwanaga J, Koba A, Natori T, et al. Three-dimensional ultrastructural analysis of the interface between an implanted demineralised dentin matrix and the surrounding newly formed bone. Sci Rep. 2018; 8:2858.
Article37. El Bialy I, Jiskoot W, Reza Nejadnik M. Formulation, delivery and stability of bone morphogenetic proteins for effective bone regeneration. Pharm Res. 2017; 34:1152–1170.
Article38. Rumpler M, Würger T, Roschger P, Zwettler E, Sturmlechner I, Altmann P, et al. Osteoclasts on bone and dentin in vitro: mechanism of trail formation and comparison of resorption behavior. Calcif Tissue Int. 2013; 93:526–539.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Demineralized dentin matrix combined with recombinant human bone morphogenetic protein-2 in rabbit calvarial defects
- Bone regeneration of demineralized dentin matrix with platelet-rich fibrin and recombinant human bone morphogenetic protein-2 on the bone defects in rabbit calvaria
- Bone Induction by Demineralized Dentin Matrix in Nude Mouse Muscles
- Complications of Anterior Cervical Fusion using a Low-dose Recombinant Human Bone Morphogenetic Protein-2
- Combined effect of recombinant human bone morphogenetic protein-2 and low level laser irradiation on bisphosphonate-treated osteoblasts