J Korean Neuropsychiatr Assoc.
2016 Aug;55(3):209-214. 10.4306/jknpa.2016.55.3.209.
Delay in Normalization of Disrupted Sleep-Wake Cycle in Mice as a Bipolar Disorder-Prone Animal Model (Bipolar Disorder-Prone Animal Model)
- Affiliations
-
- 1Department of Psychiatry, Pusan National University Hospital, Busan, Korea. jmback@pusan.ac.kr
- 2Department of Psychiatry, Pusan National University School of Medicine, Yangsan, Korea.
- KMID: 2351547
- DOI: http://doi.org/10.4306/jknpa.2016.55.3.209
Abstract
OBJECTIVES
This study was designed to test the hypothesis that delayed recovery from disrupted circadian rhythm is associated with both manic and depressive episodes in bipolar disorder.
METHODS
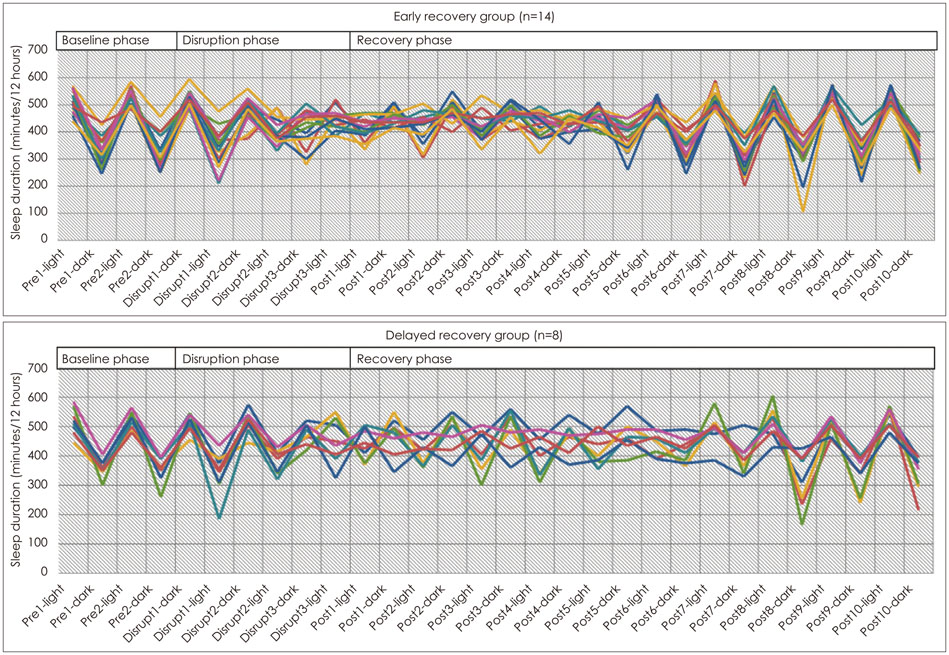
Twenty-two male mice (age of five weeks, weight 28-30 gm) underwent three days of light-dark cycle disruption and 10 days of recovery phase. Sleep and wake state were checked every five minutes during the entire experimental period. After recovery phase, quinpirole (0.5 mg/kg, s.c.) was injected into the mice and open field locomotor activities were checked. Five days after the open field test, immobility time during the last 4 min in 6 min of forced swimming test was measured. Animals which recovered sleep-wake cycle within six days after light-dark cycle disruption were assigned to the early recovery group (n=14), and those that failed to recover in six days were assigned to the delayed recovery group (n=8). The locomotor activities and the immobility times of the two groups were compared by Mann-Whiney U test at two-tailed significance level of 0.05.
RESULTS
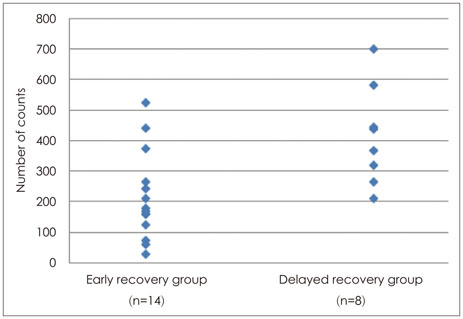
The locomotor activities of the delayed recovery group were higher (mean rank=16.19) than those of the early recovery group (mean rank=8.82, U=18.5, p=0.008). The immobility times did not differ by recovery time (U=32.0 p=0.110).
CONCLUSION
The results suggest that delayed recovery from circadian rhythm disruption raises the risk of manic symptoms rather than depressive symptoms.
MeSH Terms
Figure
Reference
-
1. Bebbington P, Ramana R. The epidemiology of bipolar affective disorder. Soc Psychiatry Psychiatr Epidemiol. 1995; 30:279–292.
Article2. Kripke DF, Mullaney DJ, Atkinson M, Wolf S. Circadian rhythm disorders in manic-depressives. Biol Psychiatry. 1978; 13:335–351.3. Winkler D, Pjrek E, Praschak-Rieder N, Willeit M, Pezawas L, Konstantinidis A, et al. Actigraphy in patients with seasonal affective disorder and healthy control subjects treated with light therapy. Biol Psychiatry. 2005; 58:331–336.
Article4. Giglio LM, Magalhães PV, Andersen ML, Walz JC, Jakobson L, Kapczinski F. Circadian preference in bipolar disorder. Sleep Breath. 2010; 14:153–155.
Article5. Wood J, Birmaher B, Axelson D, Ehmann M, Kalas C, Monk K, et al. Replicable differences in preferred circadian phase between bipolar disorder patients and control individuals. Psychiatry Res. 2009; 166:201–209.
Article6. Salvatore P, Ghidini S, Zita G, De Panfilis C, Lambertino S, Maggini C, et al. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar Disord. 2008; 10:256–265.
Article7. Millar A, Espie CA, Scott J. The sleep of remitted bipolar outpatients: a controlled naturalistic study using actigraphy. J Affect Disord. 2004; 80:145–153.
Article8. Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord. 2005; 7:176–186.
Article9. Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007; 104:6406–6411.10. Rosa AR, Comes M, Torrent C, Solè B, Reinares M, Pachiarotti I, et al. Biological rhythm disturbance in remitted bipolar patients. Int J Bipolar Disord. 2013; 1:6.
Article11. Van Dongen HP, Belenky G. Individual differences in vulnerability to sleep loss in the work environment. Ind Health. 2009; 47:518–526.
Article12. Jud C, Schmutz I, Hampp G, Oster H, Albrecht U. A guideline for analyzing circadian wheel-running behavior in rodents under different lighting conditions. Biol Proced Online. 2005; 7:101–116.
Article13. Van Dongen HP. Shift work and inter-individual differences in sleep and sleepiness. Chronobiol Int. 2006; 23:1139–1147.
Article14. Jung SH, Park JM, Moon E, Chung YI, Lee BD, Lee YM, et al. Delay in the recovery of normal sleep-wake cycle after disruption of the light-dark cycle in mice: a bipolar disorder-prone animal model? Psychiatry Investig. 2014; 11:487–491.
Article15. Souêtre E, Salvati E, Belugou JL, Pringuey D, Candito M, Krebs B, et al. Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res. 1989; 28:263–278.
Article16. Einat H, Kronfeld-Schor N, Eilam D. Sand rats see the light: short photoperiod induces a depression-like response in a diurnal rodent. Behav Brain Res. 2006; 173:153–157.
Article17. Leach G, Adidharma W, Yan L. Depression-like responses induced by daytime light deficiency in the diurnal grass rat (Arvicanthis niloticus). PLoS One. 2013; 8:e57115.
Article18. Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984; 8:269–300.
Article19. Kato T, Kubota M, Kasahara T. Animal models of bipolar disorder. Neurosci Biobehav Rev. 2007; 31:832–842.
Article20. Shaldubina A, Einat H, Szechtman H, Shimon H, Belmaker RH. Preliminary evaluation of oral anticonvulsant treatment in the quinpirole model of bipolar disorder. J Neural Transm (Vienna). 2002; 109:433–440.
Article21. Ieraci A, Mallei A, Popoli M. Social isolation stress induces anxious-depressive-like behavior and alterations of neuroplasticity-related genes in adult male mice. Neural Plast. 2016; 2016:6212983.
Article22. Viana A, do Rego JC, von Poser G, Ferraz A, Heckler AP, Costentin J, et al. The antidepressant-like effect of Hypericum caprifoliatum Cham & Schlecht (Guttiferae) on forced swimming test results from an inhibition of neuronal monoamine uptake. Neuropharmacology. 2005; 49:1042–1052.
Article23. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977; 266:730–732.
Article24. Eckeli AL, Dach F, Rodrigues AL. Acute treatments with GMP produce antidepressant-like effects in mice. Neuroreport. 2000; 11:1839–1843.
Article25. Kaster MP, Rosa AO, Rosso MM, Goulart EC, Santos AR, Rodrigues AL. Adenosine administration produces an antidepressant-like effect in mice: evidence for the involvement of A1 and A2A receptors. Neurosci Lett. 2004; 355:21–24.
Article26. Whitaker NG, Lindstrom TD. Disposition and biotransformation of quinpirole, a new D-2 dopamine agonist antihypertensive agent, in mice, rats, dogs, and monkeys. Drug Metab Dispos. 1987; 15:107–113.27. Zomkowski AD, Engel D, Gabilan NH, Rodrigues AL. Involvement of NMDA receptors and L-arginine-nitric oxide-cyclic guanosine monophosphate pathway in the antidepressant-like effects of escitalopram in the forced swimming test. Eur Neuropsychopharmacol. 2010; 20:793–801.
Article28. Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology (Berl). 2001; 155:315–322.
Article29. Lamont EW, Legault-Coutu D, Cermakian N, Boivin DB. The role of circadian clock genes in mental disorders. Dialogues Clin Neurosci. 2007; 9:333–342.
Article30. Paul MA, Love RJ, Hawton A, Arendt J. Sleep and the endogenous melatonin rhythm of high arctic residents during the summer and winter. Physiol Behav. 2015; 141:199–206.
Article31. Coomans CP, Ramkisoensing A, Meijer JH. The suprachiasmatic nuclei as a seasonal clock. Front Neuroendocrinol. 2015; 37:29–42.
Article32. Solberg LC, Olson SL, Turek FW, Redei E. Altered hormone levels and circadian rhythm of activity in the WKY rat, a putative animal model of depression. Am J Physiol Regul Integr Comp Physiol. 2001; 281:R786–R794.
Article33. Griesauer I, Diao W, Ronovsky M, Elbau I, Sartori S, Singewald N, et al. Circadian abnormalities in a mouse model of high trait anxiety and depression. Ann Med. 2014; 46:148–154.
Article34. Brocco M, Dekeyne A, Papp M, Millan MJ. Antidepressant-like properties of the anti-Parkinson agent, piribedil, in rodents: mediation by dopamine D2 receptors. Behav Pharmacol. 2006; 17:559–572.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Delay in the Recovery of Normal Sleep-Wake Cycle after Disruption of the Light-Dark Cycle in Mice: A Bipolar Disorder-Prone Animal Model?
- Bipolar Disorder, Circadian Rhythm and Clock Genes
- Is the Circadian Rhythm Dysregulation a Core Pathogenetic Mechanism of Bipolar Disorder?
- Melatonin in Psychiatric Disorders
- Sleep Disorders in Bipolar Disorders: A Narrative Review on Circadian Rhythm Disturbances and Sleep Apnoea