Yonsei Med J.
2015 Nov;56(6):1643-1650. 10.3349/ymj.2015.56.6.1643.
Comparative Effects of Ibandronate and Paclitaxel on Immunocompetent Bone Metastasis Model
- Affiliations
-
- 1Department of Endocrinology & Metabolism, Ajou University School of Medicine, Suwon, Korea.
- 2Department of Medical Biotechnology, Dongguk University, Seoul, Korea. tlee@dongguk.edu
- KMID: 2345895
- DOI: http://doi.org/10.3349/ymj.2015.56.6.1643
Abstract
- PURPOSE
Bone metastasis invariably increases morbidity and mortality. This study compares the effects of ibandronate and paclitaxel on bone structure and its mechanical properties and biochemical turnover in resorption markers using an immunocompetent Walker 256-Sprague-Dawley model, which was subjected to tumor-induced osteolysis.
MATERIALS AND METHODS
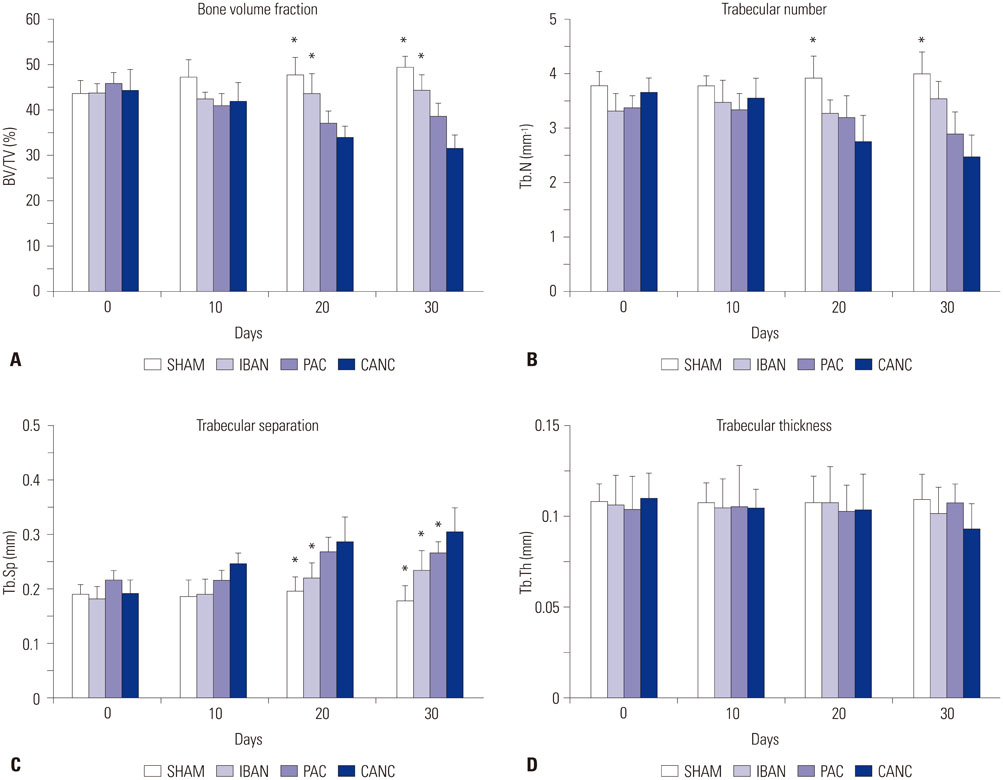
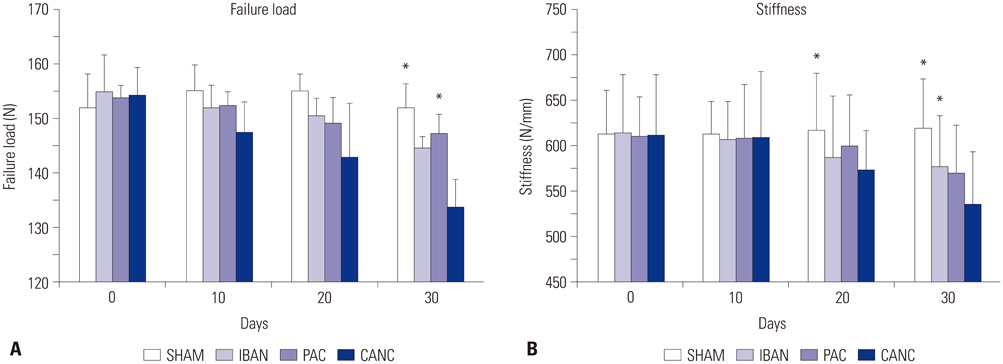
Seventy rats were divided equally into 4 groups: 1) sham group (SHAM), 2) tumor group (CANC), 3) ibandronate treated group (IBAN), and 4) paclitaxel treated group (PAC). Morphological indices [bone volume fraction (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp)] and mechanical properties (failure load, stiffness) were evaluated after thirty days of treatment period. Bone resorption rate was analysed using serum deoxypyridinoline (Dpd) concentrations.
RESULTS
Morphological indices showed that ibandronate (anti-resorptive drug) had a better effect in treating tumor-induced architectural changes in bone than paclitaxel (chemotherapeutic drug). The deterioration in bone architecture was reflected in the biomechanical properties of bone as studied with decreased failure load (F(x)) and stiffness (S) of the bone on the 30th day postsurgery. Dpd concentrations were significantly lower in the IBAN group, indicating successful inhibition of bone resorption and destruction.
CONCLUSION
Ibandronate was found to be as effective as higher doses of paclitaxel in maintaining stiffness of bone. Paclitaxel treatment did not appear to inhibit osteoclast resorption, which is contrary to earlier in-vitro literature. Emphasis should be placed on the use of immunocompetent models for examining drug efficacy since it adequately reflects bone metastasis in clinical scenarios.
MeSH Terms
-
Amino Acids
Animals
Biomechanical Phenomena/*drug effects/physiology
Bone Density/drug effects/physiology
Bone Neoplasms/*drug therapy
Bone Resorption/*chemically induced
Diphosphonates/*pharmacology
Immunocompetence
Male
*Neoplasm Metastasis
*Osteolysis
Paclitaxel/*pharmacology
Rats
Rats, Sprague-Dawley
Amino Acids
Diphosphonates
Paclitaxel
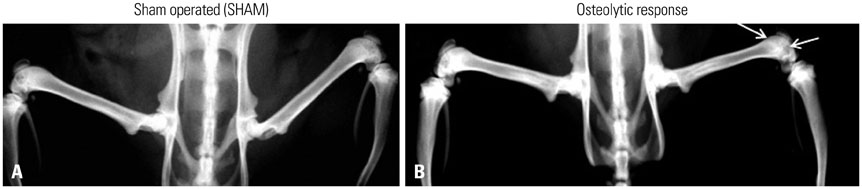
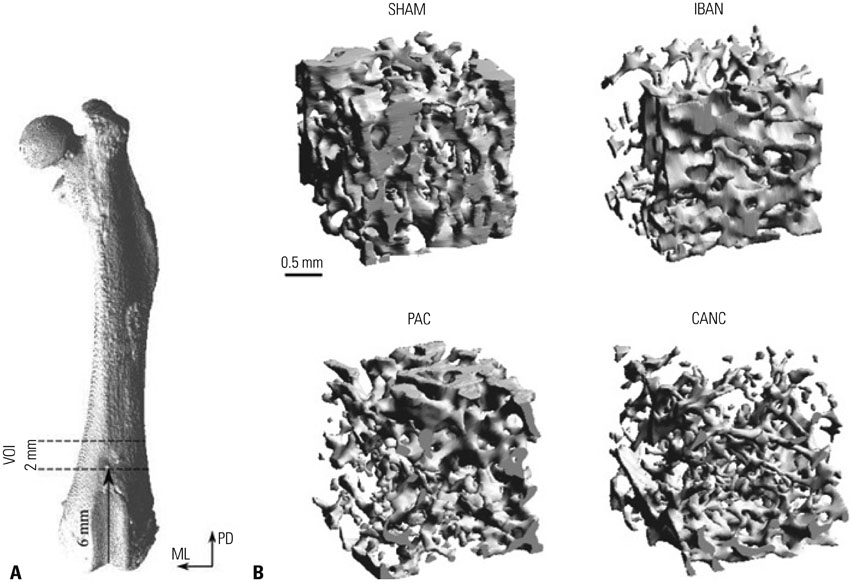
Figure
Reference
-
1. Mundy GR. Mechanisms of bone metastasis. Cancer. 1997; 80:8 Suppl. 1546–1556.
Article2. Bussard KM, Mastro AM. Ex-vivo analysis of the bone microenvironment in bone metastatic breast cancer. J Mammary Gland Biol Neoplasia. 2009; 14:387–395.3. Kurth AA, Müller R. The effect of an osteolytic tumor on the three-dimensional trabecular bone morphology in an animal model. Skeletal Radiol. 2001; 30:94–98.4. Alvarez E, Westmore M, Galvin RJ, Clapp CL, Considine EL, Smith SJ, et al. Properties of bisphosphonates in the 13762 rat mammary carcinoma model of tumor-induced bone resorption. Clin Cancer Res. 2003; 9:5705–5713.5. Bauss F, Body JJ. Ibandronate in metastatic bone disease: a review of preclinical data. Anticancer Drugs. 2005; 16:107–118.
Article6. Peltier S, Oger JM, Lagarce F, Couet W, Benoît JP. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded lipid nanocapsules. Pharm Res. 2006; 23:1243–1250.
Article7. Shord SS, Camp JR. Intravenous administration of paclitaxel in Sprague-Dawley rats: what is a safe dose? Biopharm Drug Dispos. 2006; 27:191–196.
Article8. Stearns ME, Wang M. Effects of alendronate and taxol on PC-3 ML cell bone metastases in SCID mice. Invasion Metastasis. 1996; 16:116–131.9. Heldman AW, Cheng L, Jenkins GM, Heller PF, Kim DW, Ware M Jr, et al. Paclitaxel stent coating inhibits neointimal hyperplasia at 4 weeks in a porcine model of coronary restenosis. Circulation. 2001; 103:2289–2295.10. Lalla S, Hothorn LA, Haag N, Bader R, Bauss F. Lifelong administration of high doses of ibandronate increases bone mass and maintains bone quality of lumbar vertebrae in rats. Osteoporos Int. 1998; 8:97–103.
Article11. Russell RG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008; 19:733–759.12. Muthukumaran P, Lim CT, Lee T. Estradiol influences the mechanical properties of human fetal osteoblasts through cytoskeletal changes. Biochem Biophys Res Commun. 2012; 423:503–508.
Article13. Coleman RE. Adjuvant bisphosphonates in breast cancer: are we witnessing the emergence of a new therapeutic strategy? Eur J Cancer. 2009; 45:1909–1915.
Article14. Diel IJ. Antitumour effects of bisphosphonates: first evidence and possible mechanisms. Drugs. 2000; 59:391–399.15. Hall DG, Stoica G. Effect of the bisphosphonate risedronate on bone metastases in a rat mammary adenocarcinoma model system. J Bone Miner Res. 1994; 9:221–230.
Article16. Russell RG. Ibandronate: pharmacology and preclinical studies. Bone. 2006; 38:4 Suppl 1. S7–S12.17. Kurth AA, Kim SZ, Sedlmeyer I, Bauss F, Shea M. Ibandronate treatment decreases the effects of tumor-associated lesions on bone density and strength in the rat. Bone. 2002; 30:300–306.
Article18. Kurth AA, Kim SZ, Shea M, Bauss F, Hayes WC, Müller R. Preventative ibandronate treatment has the most beneficial effect on the microstructure of bone in experimental tumor osteolysis. J Bone Miner Metab. 2007; 25:86–92.
Article19. Fan TM. Intravenous aminobisphosphonates for managing complications of malignant osteolysis in companion animals. Top Companion Anim Med. 2009; 24:151–156.
Article20. Yang X, Chan YH, Muthukumaran P, Dasde S, Teoh SH, Lee T. Ibandronate does not reduce the anabolic effects of PTH in ovariectomized rat tibiae: a microarchitectural and mechanical study. Bone. 2011; 48:1154–1163.
Article21. Arrington SA, Schoonmaker JE, Damron TA, Mann KA, Allen MJ. Temporal changes in bone mass and mechanical properties in a murine model of tumor osteolysis. Bone. 2006; 38:359–367.
Article22. Chanda D, Isayeva T, Kumar S, Siegal GP, Szafran AA, Zinn KR, et al. Systemic osteoprotegerin gene therapy restores tumor-induced bone loss in a therapeutic model of breast cancer bone metastasis. Mol Ther. 2008; 16:871–878.
Article23. Mann KA, Lee J, Arrington SA, Damron TA, Allen MJ. Predicting distal femur bone strength in a murine model of tumor osteolysis. Clin Orthop Relat Res. 2008; 466:1271–1278.
Article24. Neudert M, Fischer C, Krempien B, Bauss F, Seibel MJ. Site-specific human breast cancer (MDA-MB-231) metastases in nude rats: model characterisation and in vivo effects of ibandronate on tumour growth. Int J Cancer. 2003; 107:468–477.
Article25. Fleisch H. The bisphosphonate ibandronate, given daily as well as discontinuously, decreases bone resorption and increases calcium retention as assessed by 45Ca kinetics in the intact rat. Osteoporos Int. 1996; 6:166–170.
Article26. Chen X, Goh JC, Teoh SH, De SD, Soong R, Lee T. Localized sclerotic bone response demonstrated reduced nanomechanical creep properties. J Mech Behav Biomed Mater. 2013; 17:198–208.
Article27. Yang X, Chan YH, Muthukumaran P, Lee T. Morphological and mechanical changes in ovariectomized rat tibia with treatments of ibandronate and parathyroid hormone. Osteoporosis. 2010; 8:255–265.28. Yang X, Muthukumaran P, Lee T. Effect of ibandronate and hPTH on the viscoelastic response of ovariectomized rat femur. Osteoporosis. 2011; 9:222–230.29. Allen MJ. Biochemical markers of bone metabolism in animals: uses and limitations. Vet Clin Pathol. 2003; 32:101–113.
Article30. Jaganjac M, Poljak-Blazi M, Zarkovic K, Schaur RJ, Zarkovic N. The involvement of granulocytes in spontaneous regression of Walker 256 carcinoma. Cancer Lett. 2008; 260:180–186.
Article31. Jung A, Bornand J, Mermillod B, Edouard C, Meunier PJ. Inhibition by diphosphonates of bone resorption induced by the Walker tumor of the rat. Cancer Res. 1984; 44:3007–3011.32. Lee T. Predicting failure load of the femur with simulated osteolytic defects using noninvasive imaging technique in a simplified load case. Ann Biomed Eng. 2007; 35:642–650.
Article33. Lee T, Pereira BP, Chung YS, Oh HJ, Choi JB, Lim D, et al. Novel approach of predicting fracture load in the human proximal femur using non-invasive QCT imaging technique. Ann Biomed Eng. 2009; 37:966–975.
Article34. Lee T, Choi JB, Schafer BW, Segars WP, Eckstein F, Kuhn V, et al. Assessing the susceptibility to local buckling at the femoral neck cortex to age-related bone loss. Ann Biomed Eng. 2009; 37:1910–1920.
Article35. Strube A, Hoffmann J, Stepina E, Hauff P, Klar U, Käkönen SM. Sagopilone inhibits breast cancer bone metastasis and bone destruction due to simultaneous inhibition of both tumor growth and bone resorption. Clin Cancer Res. 2009; 15:3751–3759.
Article36. Halvorson KG, Sevcik MA, Ghilardi JR, Sullivan LJ, Koewler NJ, Bauss F, et al. Intravenous ibandronate rapidly reduces pain, neurochemical indices of central sensitization, tumor burden, and skeletal destruction in a mouse model of bone cancer. J Pain Symptom Manage. 2008; 36:289–303.
Article37. Steed H, Sawyer MB. Pharmacology, pharmacokinetics and pharmacogenomics of paclitaxel. Pharmacogenomics. 2007; 8:803–815.
Article38. Hall TJ, Jeker H, Schaueblin M. Taxol inhibits osteoclastic bone resorption. Calcif Tissue Int. 1995; 57:463–465.
Article39. Ermolaeva LA, Dubskaya TY, Fomina TI, Vetoshkina TV, Gol'dberg VE. Toxic effect of an antitumor drug paclitaxel on morphofunctional characteristics of the liver in rats. Bull Exp Biol Med. 2008; 145:263–265.
Article40. Tarumi W, Suzuki N, Takahashi N, Kobayashi Y, Kiguchi K, Sato K, et al. Ovarian toxicity of paclitaxel and effect on fertility in the rat. J Obstet Gynaecol Res. 2009; 35:414–420.
Article41. Brennan TC, Rizzoli R, Ammann P. Selective modification of bone quality by PTH, pamidronate, or raloxifene. J Bone Miner Res. 2009; 24:800–808.
Article42. Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J Clin Invest. 1997; 100:1475–1480.
Article43. Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res. 2000; 60:2949–2954.44. Fromigue O, Lagneaux L, Body JJ. Bisphosphonates induce breast cancer cell death in vitro. J Bone Miner Res. 2000; 15:2211–2221.45. Ottewell PD, Woodward JK, Lefley DV, Evans CA, Coleman RE, Holen I. Anticancer mechanisms of doxorubicin and zoledronic acid in breast cancer tumor growth in bone. Mol Cancer Ther. 2009; 8:2821–2832.46. Verdijk R, Franke HR, Wolbers F, Vermes I. Differential effects of bisphosphonates on breast cancer cell lines. Cancer Lett. 2007; 246:308–312.47. Winter MC, Holen I, Coleman RE. Exploring the anti-tumour activity of bisphosphonates in early breast cancer. Cancer Treat Rev. 2008; 34:453–475.48. Zheng Y, Zhou H, Brennan K, Blair JM, Modzelewski JR, Seibel MJ, et al. Inhibition of bone resorption, rather than direct cytotoxicity, mediates the anti-tumour actions of ibandronate and osteoprotegerin in a murine model of breast cancer bone metastasis. Bone. 2007; 40:471–478.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Ibandronate and hPTH on the Viscoelastic Response of Ovariectomized Rat Femur
- Cost-Effectiveness of Genexol-PM for Treating Metastatic Breast Cancer
- Efficacy and Safety of Monthly 150 mg Oral Ibandronate in Women with Postmenopausal Osteoporosis: A Systematic Review and Meta-analysis of Randomized Controlled Trials
- Comparative Effects of Paclitaxel and Nitric Oxide on Superficial Murine Bladder Tumor Cells
- Cost-Effectiveness of Denosumab for the Treatment of Postmenopausal Osteoporosis in South Korea