Ann Dermatol.
2016 Aug;28(4):486-490. 10.5021/ad.2016.28.4.486.
Increased Infiltration of CD8⺠T Cells by Dacarbazine in a Patient with Mucosal Penile Melanoma Refractory to Nivolumab
- Affiliations
-
- 1Department of Dermatology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan. tnamderm@tmd.ac.jp
- 2Department of Plastic Surgery, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan.
- 3Department of Pathology, Graduate School of Medical and Dental Sciences, Tokyo Medical and Dental University, Tokyo, Japan.
- KMID: 2344820
- DOI: http://doi.org/10.5021/ad.2016.28.4.486
Abstract
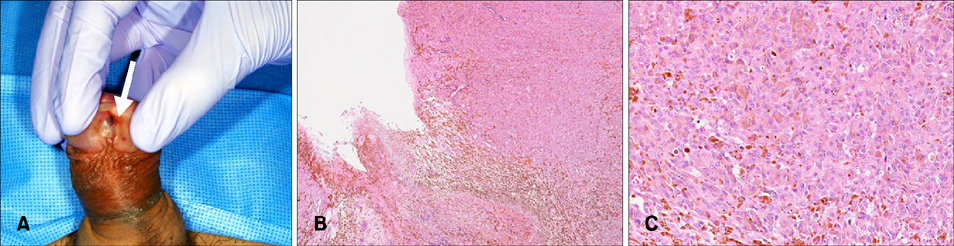
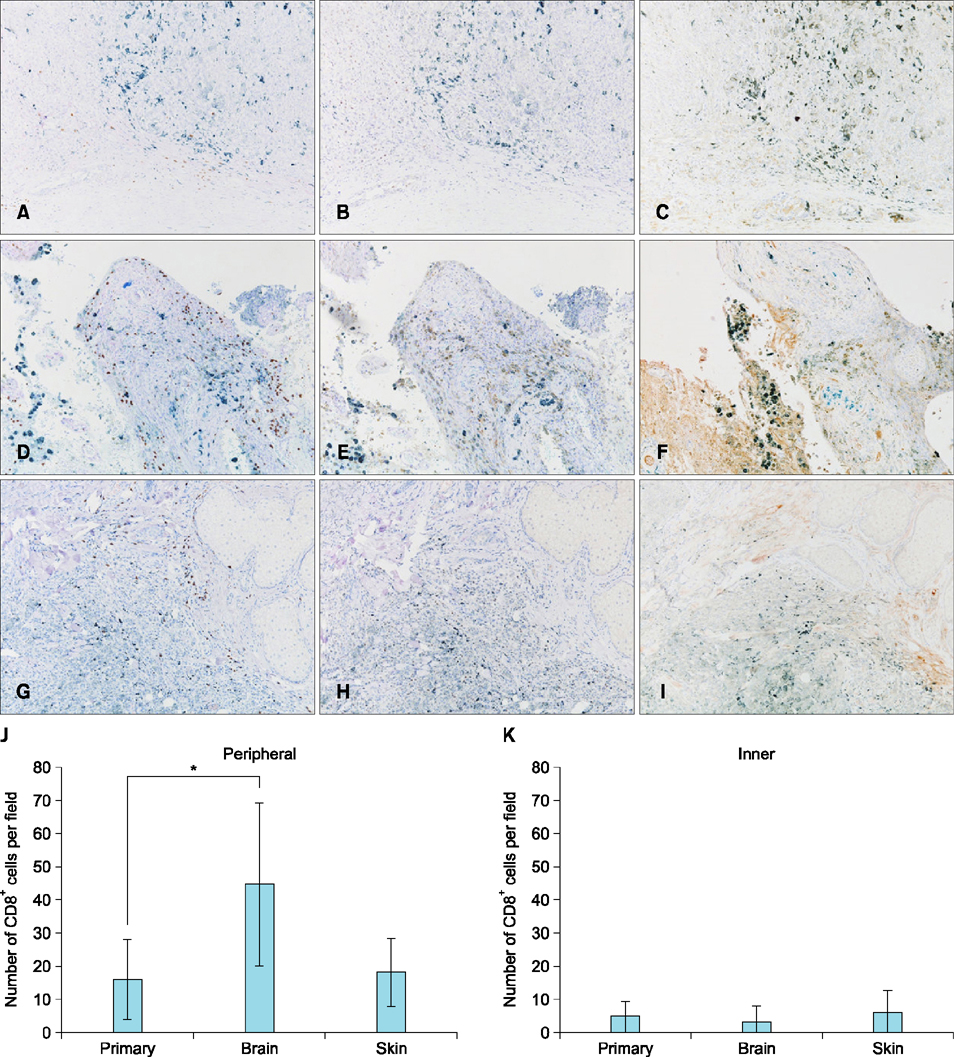
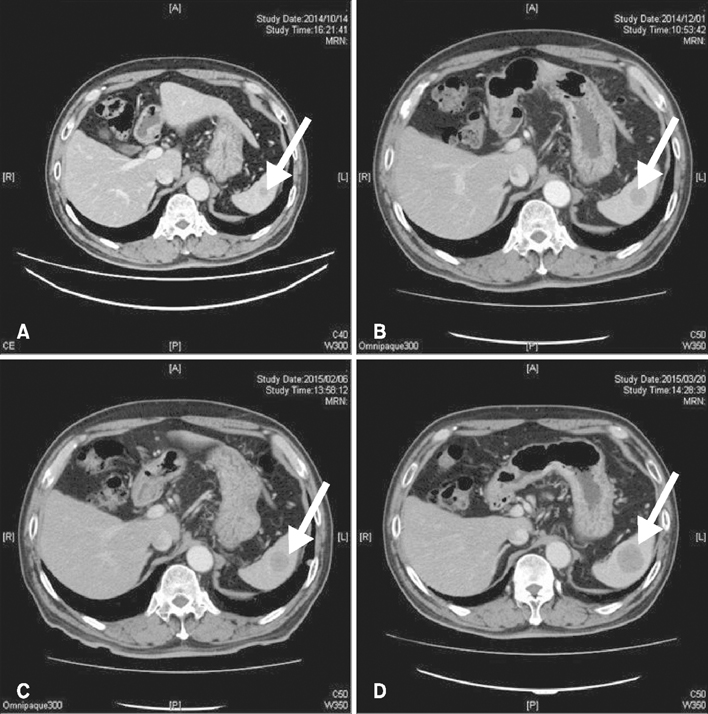
- Primary penile melanomas are rare tumors that represent less than 0.1% of all melanomas. We report a case of a 60-year-old Japanese male with a mucosal penile melanoma and describe an increased CD8⺠T cell infiltration in brain after dacarbazine (DTIC) administration. After partial penectomy and left inguinal lymphadenectomy, he developed multiple lung, bone, spleen, brain and skin metastases. He was treated with interferon-β, DTIC and nivolumab. However, the metastases were not reduced in size. Immunohistochemistry showed an increase of CD8⺠T cell infiltration and programmed death-ligand 1 (PD-L1) expression after the administration of DTIC, but the expression of programmed cell death protein 1 (PD-1) was negative. We speculate that DTIC exerted immunostimulatory effects, but nivolumab was ineffective due to the negative expression of PD-1 and/or an insufficient infiltration of CD8⺠T cells. Although this is only one case, this case report could be the first step to discuss the development of effective therapies against melanoma to take advantage of the increased CD8⺠T cell infiltration elicited by chemotherapeutic agents. It would be beneficial to pay more attention to the relationship between DTIC and immune checkpoint modulators.
Keyword
MeSH Terms
Figure
Reference
-
1. Oliva E, Quinn TR, Amin MB, Eble JN, Epstein JI, Srigley JR, et al. Primary malignant melanoma of the urethra: a clinicopathologic analysis of 15 cases. Am J Surg Pathol. 2000; 24:785–796.2. Ugurel S, Paschen A, Becker JC. Dacarbazine in melanoma: from a chemotherapeutic drug to an immunomodulating agent. J Invest Dermatol. 2013; 133:289–292.
Article3. Müller P, Martin K, Theurich S, von Bergwelt-Baildon M, Zippelius A. Cancer chemotherapy agents target intratumoral dendritic cells to potentiate antitumor immunity. Oncoimmunology. 2014; 3:e954460.
Article4. Hervieu A, Rébé C, Végran F, Chalmin F, Bruchard M, Vabres P, et al. Dacarbazine-mediated upregulation of NKG2D ligands on tumor cells activates NK and CD8 T cells and restrains melanoma growth. J Invest Dermatol. 2013; 133:499–508.
Article5. Hirano F, Kaneko K, Tamura H, Dong H, Wang S, Ichikawa M, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005; 65:1089–1096.6. Ozawa A, Nomiyama T, Nakai N, Hartmann G, Takenaka H, Kishimoto S, et al. Immunohistological analysis of in-transit metastasis in a patient with advanced melanoma treated with combination therapy of cytosine guanine dinucleotide oligodeoxynucleotide, dacarbazine and beta-interferon: a case report. J Dermatol. 2012; 39:1035–1037.
Article7. Nardin A, Wong WC, Tow C, Molina TJ, Tissier F, Audebourg A, et al. Dacarbazine promotes stromal remodeling and lymphocyte infiltration in cutaneous melanoma lesions. J Invest Dermatol. 2011; 131:1896–1905.
Article8. Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med. 2012; 4:127ra37.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Lichenoid Drug Eruption Developed in Melanoma Patient Treated with Nivolumab
- A Curious Case of Primary Gastric Mucosal Melanoma
- The Major Role of NF-κB in the Depth of Invasion on Acral Melanoma by Decreasing CD8⺠T Cells
- Extrinsic Acquisition of CD80 by Antigen-Specific CD8⺠T Cells Regulates Their Recall Immune Responses to Acute Viral Infection
- Untrastructure of Melanocyte in Penile Melanosis