J Korean Soc Radiol.
2011 Nov;65(5):465-472.
Differential Diagnosis of Lymphadenopathy in the Neck Spaces in a CT Perfusion Study
- Affiliations
-
- 1Department of Radiology, Soonchunhyang University College of Medicine, Bucheon Hospital, Bucheon, Korea. hshong@schbc.ac.kr
- 2Department of Nuclear Medicine, Soonchunhyang University College of Medicine, Bucheon Hospital, Bucheon, Korea.
Abstract
- PURPOSE
To evaluate the CT perfusion parameters for differentiating between a benign and malignant lymphadenopathy in the neck spaces.
MATERIALS AND METHODS
Seventeen patients with cervical lymphadenopathy underwent perfusion CT. Perfusion parameters, including blood flow (BF), blood volume (BV), permeability index (PI), and mean transit time, were calculated at the regions of interest in the enlarged lymph nodes (LNs). The enlarged LNs were diagnosed by fine needle aspiration, surgical excision, and clinical follow-up. The LNs were classified as either reactive hyperplasia (n = 26), metastatic LNs (n = 11), or LNs of lymphoma (n = 14).
RESULTS
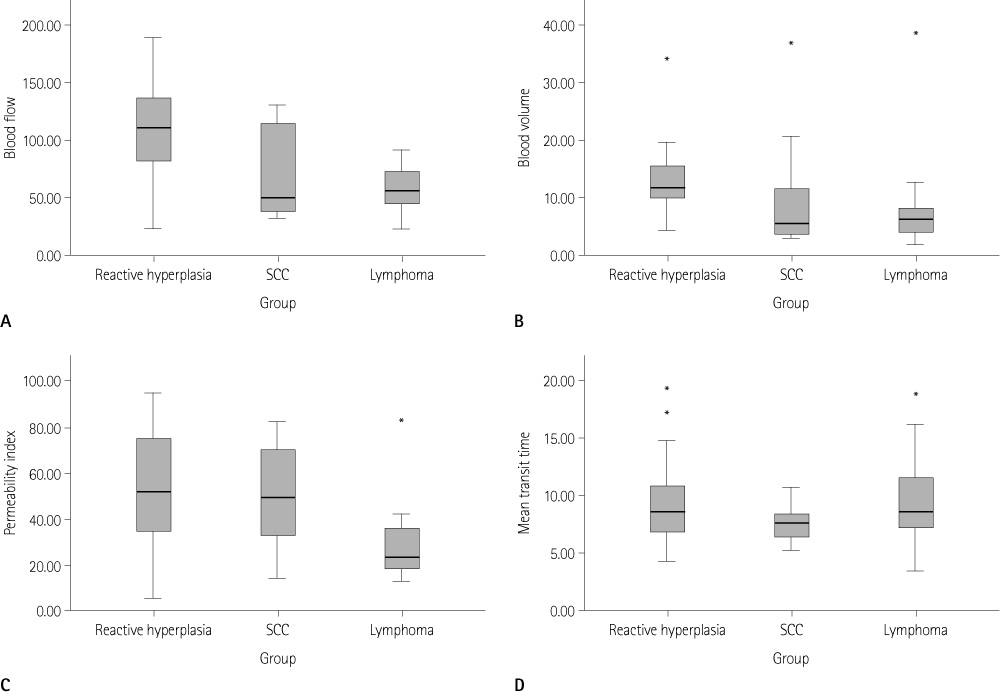
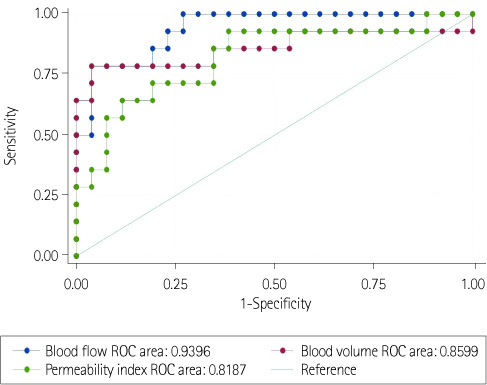
Significant differences were found for BF, BV, and PI among the three groups (p < 0.05). Reactive hyperplasia had a significantly higher BF than metastatic LN (p < 0.0167). The LNs of lymphoma had a significantly lower BF, BV, and PI than reactive hyperplasia (p < 0.0167). No significant difference was observed between the metastatic LNs of the head and neck cancer and LNs of lymphoma for all perfusion parameters.
CONCLUSION
In patients with head and neck cancer, perfusion CT is not useful for differentiating between metastatic LNs and inflammatory reactive hyperplasia. However, perfusion CT can be useful for differentiating between LNs of lymphoma and reactive hyperplasia.
MeSH Terms
Figure
Reference
-
1. van den Brekel MW, Stel HV, Castelijns JA, Nauta JJ, van der Waal I, Valk J, et al. Cervical lymph node metastasis: assessment of radiologic criteria. Radiology. 1990; 177:379–384.2. Ahuja A, Ying M, Yang WT, Evans R, King W, Metreweli C. The use of sonography in differentiating cervical lymphomatous lymph nodes from cervical metastatic lymph nodes. Clin Radiol. 1996; 51:186–190.3. Tschammler A, Ott G, Schang T, Seelbach-Goebel B, Schwager K, Hahn D. Lymphadenopathy: differentiation of benign from malignant disease--color Doppler US assessment of intranodal angioarchitecture. Radiology. 1998; 208:117–123.4. Miles KA. Tumour angiogenesis and its relation to contrast enhancement on computed tomography: a review. Eur J Radiol. 1999; 30:198–205.5. Axel L. Cerebral blood flow determination by rapid-sequence computed tomography: theoretical analysis. Radiology. 1980; 137:679–686.6. Faggioni L, Neri E, Bartolozzi C. CT perfusion of head and neck tumors: how we do it. AJR Am J Roentgenol. 2010; 194:62–69.7. Rumboldt Z, Al-Okaili R, Deveikis JP. Perfusion CT for head and neck tumors: pilot study. AJNR Am J Neuroradiol. 2005; 26:1178–1185.8. Yoon DY, Hwang HS, Chang SK, Rho YS, Ahn HY, Kim JH, et al. CT, MR, US, 18F-FDG PET/CT, and their combined use for the assessment of cervical lymph node metastases in squamous cell carcinoma of the head and neck. Eur Radiol. 2009; 19:634–642.9. Abdel Razek AA, Gaballa G. Role of perfusion magnetic resonance imaging in cervical lymphadenopathy. J Comput Assist Tomogr. 2011; 35:21–25.10. Bisdas S, Baghi M, Smolarz A, Pihno NC, Lehnert T, Knecht R, et al. Quantitative measurements of perfusion and permeability of oropharyngeal and oral cavity cancer, recurrent disease, and associated lymph nodes using first-pass contrast-enhanced computed tomography studies. Invest Radiol. 2007; 42:172–179.11. Gandhi D, Hoeffner EG, Carlos RC, Case I, Mukherji SK. Computed tomography perfusion of squamous cell carcinoma of the upper aerodigestive tract. Initial results. J Comput Assist Tomogr. 2003; 27:687–693.12. Ash L, Teknos TN, Gandhi D, Patel S, Mukherji SK. Head and neck squamous cell carcinoma: CT perfusion can help noninvasively predict intratumoral microvessel density. Radiology. 2009; 251:422–428.13. Liao W, Liu Y, Wang X, Jiang X, Tang B, Fang J, et al. Differentiation of primary central nervous system lymphoma and high-grade glioma with dynamic susceptibility contrast-enhanced perfusion magnetic resonance imaging. Acta Radiol. 2009; 50:217–225.14. Schramm P, Xyda A, Klotz E, Tronnier V, Knauth M, Hartmann M. Dynamic CT perfusion imaging of intra-axial brain tumours: differentiation of high-grade gliomas from primary CNS lymphomas. Eur Radiol. 2010; 20:2482–2490.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Regional Cortical Hyperperfusion on Perfusion CT during Postictal Motor Deficit: A Case Report
- CT Analysis of Retropharyngeal Abnormality in Kawasaki Disease
- Kikuchi Disease in the Neck: CT and US Appearances
- CT Findings of Cervical Lymphadenopathy: Differential Diagnosis
- Odontogenic Versus Nonodontogenic Deep Neck Space Infections: CT Manifestations