Cancer Res Treat.
2016 Jul;48(3):962-969. 10.4143/crt.2015.173.
Delay of Treatment Initiation Does Not Adversely Affect Survival Outcome in Breast Cancer
- Affiliations
-
- 1Department of Surgery, Seoul National University College of Medicine, Seoul, Korea. hanw@snu.ac.kr
- 2Laboratory of Breast Cancer Biology, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2344069
- DOI: http://doi.org/10.4143/crt.2015.173
Abstract
- PURPOSE
Previous studies examining the relationship between time to treatment and survival outcome in breast cancer have shown inconsistent results. The aim of this study was to analyze the overall impact of delay of treatment initiation on patient survival and to determine whether certain subgroups require more prompt initiation of treatment.
MATERIALS AND METHODS
This study is a retrospective analysis of stage I-III patients who were treated in a single tertiary institution between 2005 and 2008. Kaplan-Meier survival analysis and Cox proportional hazards regression model were used to evaluate the impact of interval between diagnosis and treatment initiation in breast cancer and various subgroups.
RESULTS
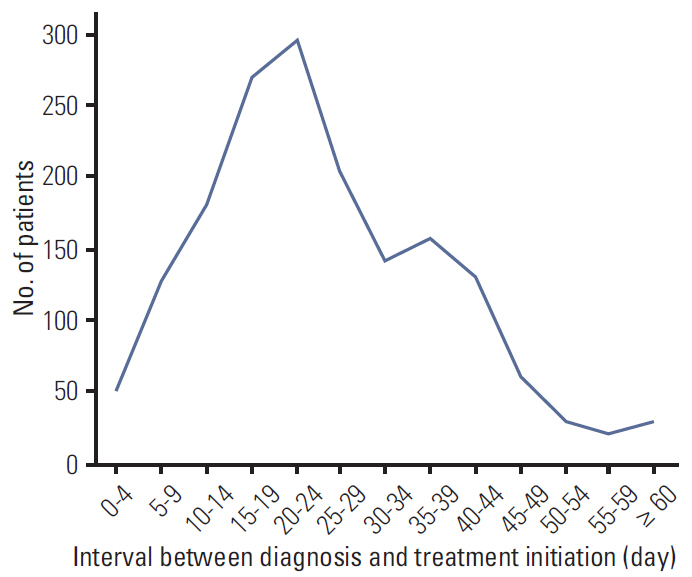
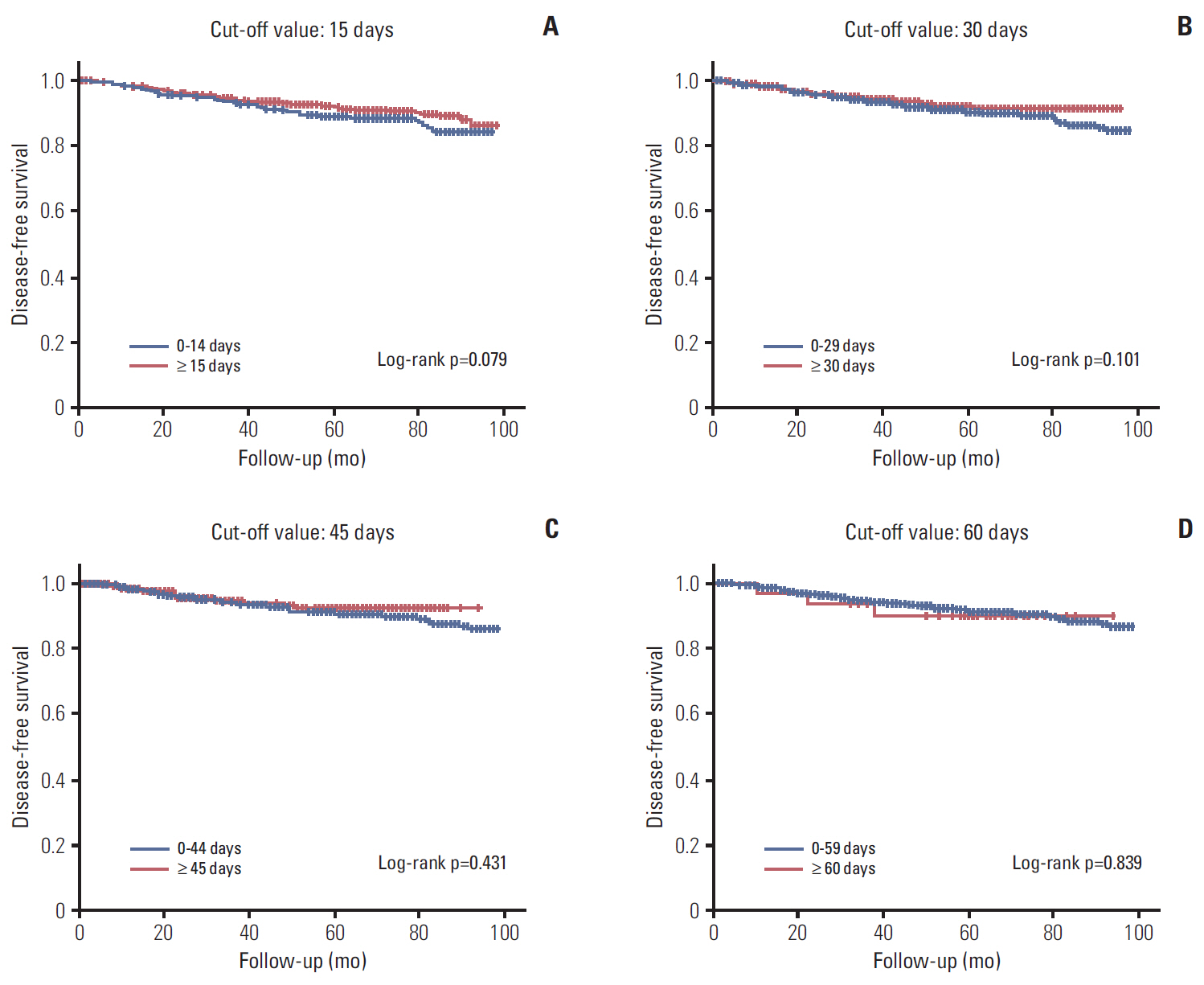
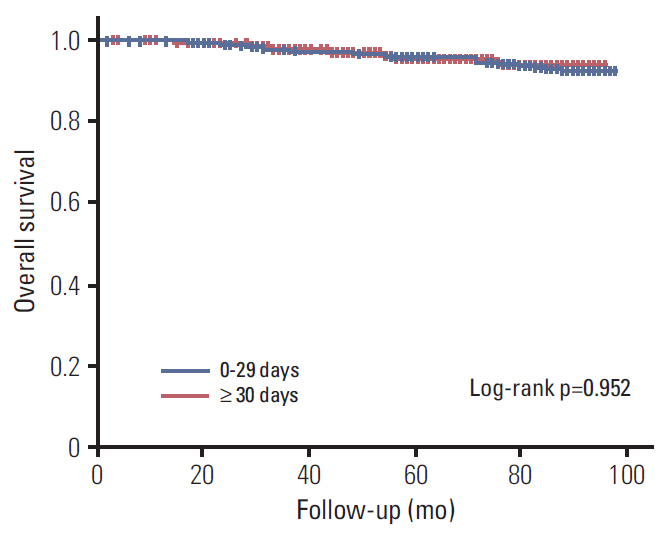
A total of 1,702 patients were included. Factors associated with longer delay of treatment initiation were diagnosis at another hospital, medical comorbidities, and procedures performed before admission for surgery. An interval between diagnosis and treatment initiation as a continuous variable or with a cutoff value of 15, 30, 45, and 60 days had no impact on disease-free survival (DFS). Subgroup analyses for hormone-responsiveness, triple-negative breast cancer, young age, clinical stage, and type of initial treatment showed no significant association between longer delay of treatment initiation and DFS.
CONCLUSION
Our results show that an interval between diagnosis and treatment initiation of 60 days or shorter does not appear to adversely affect DFS in breast cancer.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Patient and Care Delays of Breast Cancer in China
Yue-Lin Li, Ya-Chao Qin, Lu-Ying Tang, Yu-Huang Liao, Wei Zhang, Xiao-Ming Xie, Qiang Liu, Ying Lin, Ze-Fang Ren
Cancer Res Treat. 2019;51(3):1098-1106. doi: 10.4143/crt.2018.386.
Reference
-
References
1. Brazda A, Estroff J, Euhus D, Leitch AM, Huth J, Andrews V, et al. Delays in time to treatment and survival impact in breast cancer. Ann Surg Oncol. 2010; 17 Suppl 3:291–6.
Article2. McLaughlin JM, Anderson RT, Ferketich AK, Seiber EE, Balkrishnan R, Paskett ED. Effect on survival of longer intervals between confirmed diagnosis and treatment initiation among low-income women with breast cancer. J Clin Oncol. 2012; 30:4493–500.
Article3. Mujar M, Dahlui M, Yip CH, Taib NA. Delays in time to primary treatment after a diagnosis of breast cancer: does it impact survival? Prev Med. 2013; 56:222–4.
Article4. Shin DW, Cho J, Kim SY, Guallar E, Hwang SS, Cho B, et al. Delay to curative surgery greater than 12 weeks is associated with increased mortality in patients with colorectal and breast cancer but not lung or thyroid cancer. Ann Surg Oncol. 2013; 20:2468–76.
Article5. Smith EC, Ziogas A, Anton-Culver H. Delay in surgical treatment and survival after breast cancer diagnosis in young women by race/ethnicity. JAMA Surg. 2013; 148:516–23.
Article6. Yun YH, Kim YA, Min YH, Park S, Won YJ, Kim DY, et al. The influence of hospital volume and surgical treatment delay on long-term survival after cancer surgery. Ann Oncol. 2012; 23:2731–7.
Article7. Ganz PA. Quality of life across the continuum of breast cancer care. Breast J. 2000; 6:324–30.
Article8. Eastman A, Tammaro Y, Moldrem A, Andrews V, Huth J, Euhus D, et al. Outcomes of delays in time to treatment in triple negative breast cancer. Ann Surg Oncol. 2013; 20:1880–5.
Article9. Birkmeyer JD, Siewers AE, Marth NJ, Goodman DC. Regionalization of high-risk surgery and implications for patient travel times. JAMA. 2003; 290:2703–8.
Article10. Simunovic M, Theriault ME, Paszat L, Coates A, Whelan T, Holowaty E, et al. Using administrative databases to measure waiting times for patients undergoing major cancer surgery in Ontario, 1993-2000. Can J Surg. 2005; 48:137–42.11. Stitzenberg KB, Sigurdson ER, Egleston BL, Starkey RB, Meropol NJ. Centralization of cancer surgery: implications for patient access to optimal care. J Clin Oncol. 2009; 27:4671–8.
Article12. Bardell T, Belliveau P, Kong W, Mackillop WJ. Waiting times for cancer surgery in Ontario: 1984-2000. Clin Oncol (R Coll Radiol). 2006; 18:401–9.
Article13. Bilimoria KY, Ko CY, Tomlinson JS, Stewart AK, Talamonti MS, Hynes DL, et al. Wait times for cancer surgery in the United States: trends and predictors of delays. Ann Surg. 2011; 253:779–85.14. Kawakami J, Hopman WM, Smith-Tryon R, Siemens DR. Measurement of surgical wait times in a universal health care system. Can Urol Assoc J. 2008; 2:597–603.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Impact of Physician Delay on Postoperative Outcome of Patients with Acute Appendicitis
- Pregnancy After Breast Cancer – Prognostic Safety and Pregnancy Outcomes According to Oestrogen Receptor Status: A Systematic Review
- Safe and successful pregnancy following breast cancer treatment in young patients 35 years old or under without invasive fertility preservation: a retrospective study
- Breast Cancer During Pregnancy
- Translational Regulation: A Novel Target for Breast Cancer Therapy