J Korean Ophthalmol Soc.
2012 Feb;53(2):238-245.
Contrast Sensitivity and Color Vision Comparison Between Clear and Yellow-Tinted Intraocular Lens in Diabetic Retinopathy
- Affiliations
-
- 1Department of Ophthalmology, Seoul Paik Hospital, Inje University College of Medicine, Seoul, Korea.
- 2Department of Ophthalmology, Sanggye Paik Hospital, Inje University College of Medicine, Seoul, Korea. joohlee@paik.ac.kr
Abstract
- PURPOSE
To compare contrast sensitivity and color vision after implantation of a clear intraocular lens (IOL) and a yellow-tinted IOL in diabetic retinopathy patients.
METHODS
In the 50 eyes of 25 diabetic patients with non-proliferative diabetic retinopathy, clear IOLs were implanted in 25 eyes, and yellow-tinted IOLs were implanted in 25 fellow eyes. Three months after the surgery, contrast sensitivity function was measured with a vision contrast test system, and color discrimination was tested using the Farnsworth Munsell 100-hue test.
RESULTS
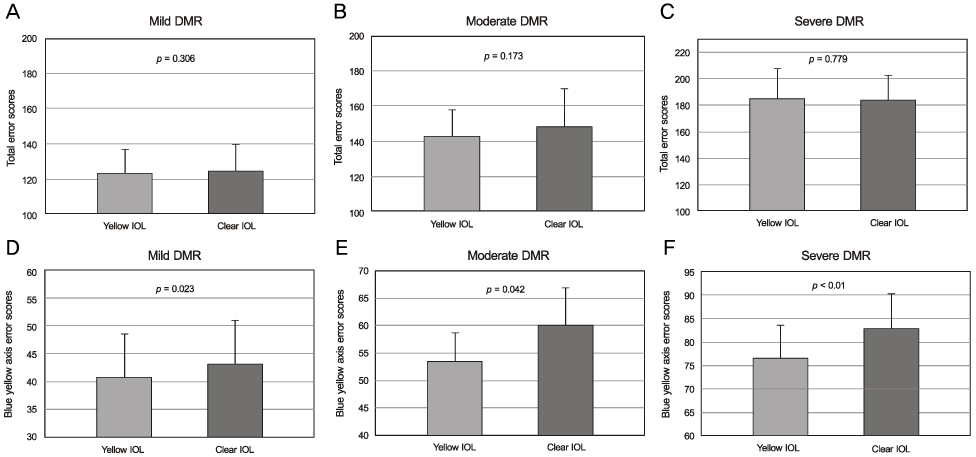
Eyes implanted with yellow-tinted IOLs had significant differences in contrast sensitivity values compared to those of fellow eyes implanted with clear IOLs in both the moderate diabetic retinopathy group (6.0 cycles per degree) and the severe diabetic retinopathy group (throughout all spatial frequencies except 12.0 cycles per degree). The yellow-tinted IOL did not modify chromatic discrimination compared with that of the clear IOL. In the blue-yellow axis error score, however, there were significant differences between the clear IOL and the yellow-tinted IOL.
CONCLUSIONS
With progressing diabetic retinopathy, the yellow-tinted IOL provided better contrast sensitivity than the clear IOL. The yellow-tinted IOL improved color vision in the blue-yellow chromatic axis without causing chromatic discrimination defects.
Keyword
MeSH Terms
Figure
Reference
-
1. Comerford JP. Vision evaluation using contrast sensitivity functions. Am J Optom Physiol Opt. 1983. 60:394–398.2. Campbell FW, Green DG. Optical and retinal factors affecting visual resolution. J Physiol. 1965. 181:576–593.3. Swanson WH, Cohen JM. Color vision. Ophthalmol Clin North Am. 2003. 16:179–203.4. Davies N, Morland A. Extent of foveal tritanopia in diabetes mellitus. Br J Ophthalmol. 2003. 87:742–746.5. Volbrecht VJ, Schneck ME, Adams AJ, et al. Diabetic short-wavelength sensitivity: variations with induced changes in blood glucose level. Invest Ophthalmol Vis Sci. 1994. 35:1243–1246.6. Thompson DG, Howarth F, Levy IS. Colour blindness, a hazard to diabetics. Lancet. 1978. 1:44.7. Thompson DG, Howarth F, Taylor H, et al. Defective colour vision in diabetes: a hazard to management. Br Med J. 1979. 1:859–860.8. Francois J, Verriest G. Acquired dyschromatopsia. Ann Ocul (Paris). 1957. 190:812–859.9. Cho NC, Poulsen GL, Ver Hoeve JN, Nork TM. Selective loss of S-cones in diabetic retinopathy. Arch Ophthalmol. 2000. 118:1393–1400.10. Gastaud P, Vola J, Saracco JB, et al. Verriest G. Diabetic dyschromatopsia: pathogenetic hypothesis. Colour Vision Deficiencies VIII. 1987. Dordrecht: Martinus Nijhoff/Dr W. Junk Publishers;387–390.11. Hood DC, Benimoff NI, Greenstein VC. The response range of the blue-cone pathways: a source of vulnerability to disease. Invest Ophthalmol Vis Sci. 1984. 25:864–867.12. Mollon JD. Verriest G, editor. What is odd about the short-wavelength mechanism and why is it disproportionately vulnerable to acquired damage? Report of a discussion. Colour Vision Deficiencies VI. 1982. The Hague: Dr W. Junk Publishers;145–149.13. Farnsworth D. Tritanomalous vision as a threshold function. 1956. New London, Conn: U.S. Naval Medical Research Laboratory, Submarine Base;185–197.14. Greenstein VC, Hood DC, Campbell CJ. The use of a flash - on - flash paradigm to assess sensitivity changes due to retinal disease. Invest Ophthalmol Vis Sci. 1982. 23:102–112.15. Nork TM, Millecchia LL, Strickland BD, et al. Selective loss of blue cones and rods in human retinal detachment. Arch Ophthalmol. 1995. 113:1066–1073.16. Kessel L, Alsing A, Larsen M. Diabetic versus non-diabetic colour vision after cataract surgery. Br J Ophthalmol. 1999. 83:1042–1045.17. Ayed S, Jeddi A, Kallal Z. Diabetes and color vision disorder detected by the Farnsworth 100 Hue test. Diabetic dyschromatopsia. J Fr Ophtalmol. 1990. 13:506–510.18. Utku D, Atmaca LS. Farnsworth-Munsell 100-hue test for patients with diabetes mellitus. Ann Ophthalmol. 1992. 24:205–208.19. Muntoni S, Serra A, Mascia C, Songini M. Dyschromatopsia in diabetes mellitus and its relation to metabolic control. Diabetes Care. 1982. 5:375–378.20. Trick GL, Burde RM, Gordon MO, et al. The relationship between hue discrimination and contrast sensitivity deficits in patients with diabetes mellitus. Ophthalmology. 1988. 95:693–698.21. Roy MS, Gunkel RD, Podgor MJ. Color vision defects in early diabetic retinopathy. Arch Ophthalmol. 1986. 104:225–228.22. Hardy KJ, Lipton J, Scase MO, et al. Detection of colour vision abnormalities in uncomplicated type 1 diabetic patients with angiographically normal retinas. Br J Ophthalmol. 1992. 76:461–464.23. Green FD, Ghafour IM, Allan D, et al. Colour vision of diabetics. Br J Ophthalmol. 1985. 69:533–536.24. Bresnick GH, Condit RS, Palta M, et al. Association of hue discrimination loss and diabetic retinopathy. Arch Ophthalmol. 1985. 103:1317–1324.25. Andley U. Albert DM, Jakobiec FA, editors. Photooxidative stress. Principles and Practice of Ophthalmology: Clinical Practice. 1994. Philadelphia: WB Saunders;417–436.26. Lanum J. The damaging effects of light on the retina. Empirical findings, theoretical and practical implications. Surv Ophthalmol. 1978. 22:221–249.27. Yoon HM, Jang Y, Kim JS, Ji NC. An ultrastructural study of recovery of photoreceptor layer from visible light-induced damage. J Korean Ophthalmol Soc. 1993. 34:678–686.28. Ham WT Jr, Mueller HA, Sliney DH. Retinal sensitivity to damage from short wavelength light. Nature. 1976. 260:153–155.29. Sperling HG, Johnson C, Harwerth RS. Differential spectral photic damage to primate cones. Vision Res. 1980. 20:1117–1125.30. Lawwill T. Three major pathologic processes caused by light in the primate retina: a search for mechanisms. Trans Am Ophthalmol Soc. 1982. 80:517–579.31. Ishida M, Sato H, Yanashima K, et al. Improving contrast sensitivity with the UVCY (Hoya) intraocular lens under glare conditions. Folia Ophthalmol Jpn. 1993. 44:399–405.32. Ham WT Jr, Mueller HA, Ruffolo JJ Jr, et al. Basic mechanisms underlying the production of photochemical lesions in the mammalian retina. Curr Eye Res. 1984. 3:165–174.33. Ham WT Jr, Ruffolo JJ Jr, Mueller HA, et al. Histologic analysis of photochemical lesions produced in rhesus retina by short-wavelength light. Invest Ophthalmol Vis Sci. 1978. 17:1029–1035.34. Sparrow JR, Miller AS, Zhou J. Blue light-absorbing intraocular lens and retinal pigment epithelium protection in vitro. J Cataract Refract Surg. 2004. 30:873–878.35. Yap M. The effect of a yellow filter on contrast sensitivity. Ophthalmic Physiol Opt. 1984. 4:227–232.36. Kinney JA, Schlichting CL, Neri DF, Kindness SW. Reaction time to spatial frequencies using yellow and luminance-matched neutral goggles. Am J Optom Physiol Opt. 1983. 60:132–138.37. Kelly SA. Effect of yellow-tinted lenses on brightness. J Opt Soc Am A. 1990. 7:1905–1911.38. Sivak JG, Bobier WR. Effect of a yellow ocular filter on chromatic aberration: the fish eye as an example. Am J Optom Physiol Opt. 1978. 55:813–817.39. Wolffsohn JS, Cochrane AL, Khoo H, et al. Contrast is enhanced by yellow lenses because of selective reduction of short-wavelength light. Optom Vis Sci. 2000. 77:73–81.40. Kuyk TK, Thomas SR. Effect of short wavelength absorbing filters on Farnsworth-Munsell 100 Hue test and hue identification task performance. Optom Vis Sci. 1990. 67:522–531.41. de Fez D, Luque MJ, Viqueira V. Enhancement of contrast sensitivity and losses of chromatic discrimination with tinted lenses. Optom Vis Sci. 2002. 79:590–597.42. Early Treatment Diabetic Retinopathy Study Research Group. Fundus photographic risk factors for progression of diabetic retinopathy. ETDRS Report number 12. Ophthalmology. 1991. 98:5 Suppl. 823–833.43. Della Sala S, Bertoni G, Somazzi L, et al. Impaired contrast sensitivity in diabetic patients with and without retinopathy: a new technique for rapid assessment. Br J Ophthalmol. 1985. 69:136–142.44. Sokol S, Moskowitz A, Skarf B, et al. Contrast sensitivity in diabetics with and without background retinopathy. Arch Ophthalmol. 1985. 103:51–54.45. Ghafour IM, Foulds WS, Allan D, McClure E. Contrast sensitivity in diabetic subjects with and without retinopathy. Br J Ophthalmol. 1982. 66:492–495.46. Trick GL, Burde RM, Gordon MO, et al. The relationship between hue discrimination and contrast sensitivity deficits in patients with diabetes mellitus. Ophthalmology. 1988. 95:693–698.47. Kim HG, Yoo CS, Huh W. Hue discrimination and contrast sensitivity deficits in diabetic subjects with and without retinopathy. J Korean Ophthalmol Soc. 1991. 32:274–280.48. Arend O, Remky A, Evans D, et al. Contrast sensitivity loss is coupled with capillary dropout in patients with diabetes. Invest Ophthalmol Vis Sci. 1997. 38:1819–1824.49. Rodríguez-Galietero A, Montés-Micó R, Muñoz G, Albarrán-Diego C. Blue-light filtering intraocular lens in patients with diabetes: contrast sensitivity and chromatic discrimination. J Cataract Refract Surg. 2005. 31:2088–2092.50. Mester U, Holz F, Kohnen T, et al. Intraindividual comparison of a blue-light filter on visual function: AF-1 (UY) versus AF-1 (UV) intraocular lens. J Cataract Refract Surg. 2008. 34:608–615.51. Neumaier-Ammerer B, Felke S, Hagen S, et al. Comparison of visual performance with blue light-filtering and ultraviolet light-filtering intraocular lenses. J Cataract Refract Surg. 2010. 36:2073–2079.52. Wang H, Wang J, Fan W, Wang W. Comparison of photochromic, yellow, and clear intraocular lenses in human eyes under photopic and mesopic lighting conditions. J Cataract Refract Surg. 2010. 36:2080–2086.53. Ao M, Chen X, Huang C, et al. Color discrimination by patients with different types of light-filtering intraocular lenses. J Cataract Refract Surg. 2010. 36:389–395.54. Ernest PH. Light-transmission-spectrum comparison of foldable intraocular lenses. J Cataract Refract Surg. 2004. 30:1755–1758.55. van den Berg TJ. Light scattering by donor lenses as a function of depth and wavelength. Invest Ophthalmol Vis Sci. 1997. 38:1321–1332.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hue Discrimination and Contrast Sensitivity Deficits in Diabetic Subjects With and Without Retinopathy
- Intraindividual Comparison of Visual Outcomes between Blue Light-filtering and Ultraviolet Light-filtering Intraocular Lens
- A Study on the Acquired Color Vision Deficit in Diabetes Mellitus
- The Effect of Yellow Tinted Intraocular Lenses on the Result of Frequency Doubling Perimetry after Cataract Surgery
- Effect of Intraocular Lens Implantation on Electro retinogram