J Korean Ophthalmol Soc.
2011 Nov;52(11):1344-1350.
Effects of Low-Dose Cyclosporine on Human Corneal Epithelial Cells
- Affiliations
-
- 1Department of Ophthalmology, Pusan National University School of Medicine, Busan, Korea. jongsool@pusan.ac.kr
- 2Department of of Ophthalmology, Maryknoll Medical Center, Busan, Korea.
Abstract
- PURPOSE
To evaluate the response and cellular changes of cultured human corneal epithelium according to the concentration of topical cyclosporine.
METHODS
Human corneal epithelial cells were exposed to cyclosporine A concentrations of 1 ug/ml (0.0001%), 10 ug/ml (0.001%), 100 ug/ml (0.01%), and 500 ug/ml (0.05%) for 5 and 10 minutes. An MTT-based colorimetric assay was performed to assess the metabolic activity of cellular proliferation and a lactate dehydrogenase (LDH) leakage assay was used to determine cellular toxicity. The modulations of extracellular matrix proteins such as PIP and laminin were evaluated. The levels of pro-inflammatory cytokine, TNF-alpha, and IL-1 were evaluated by ELISA kits.
RESULTS
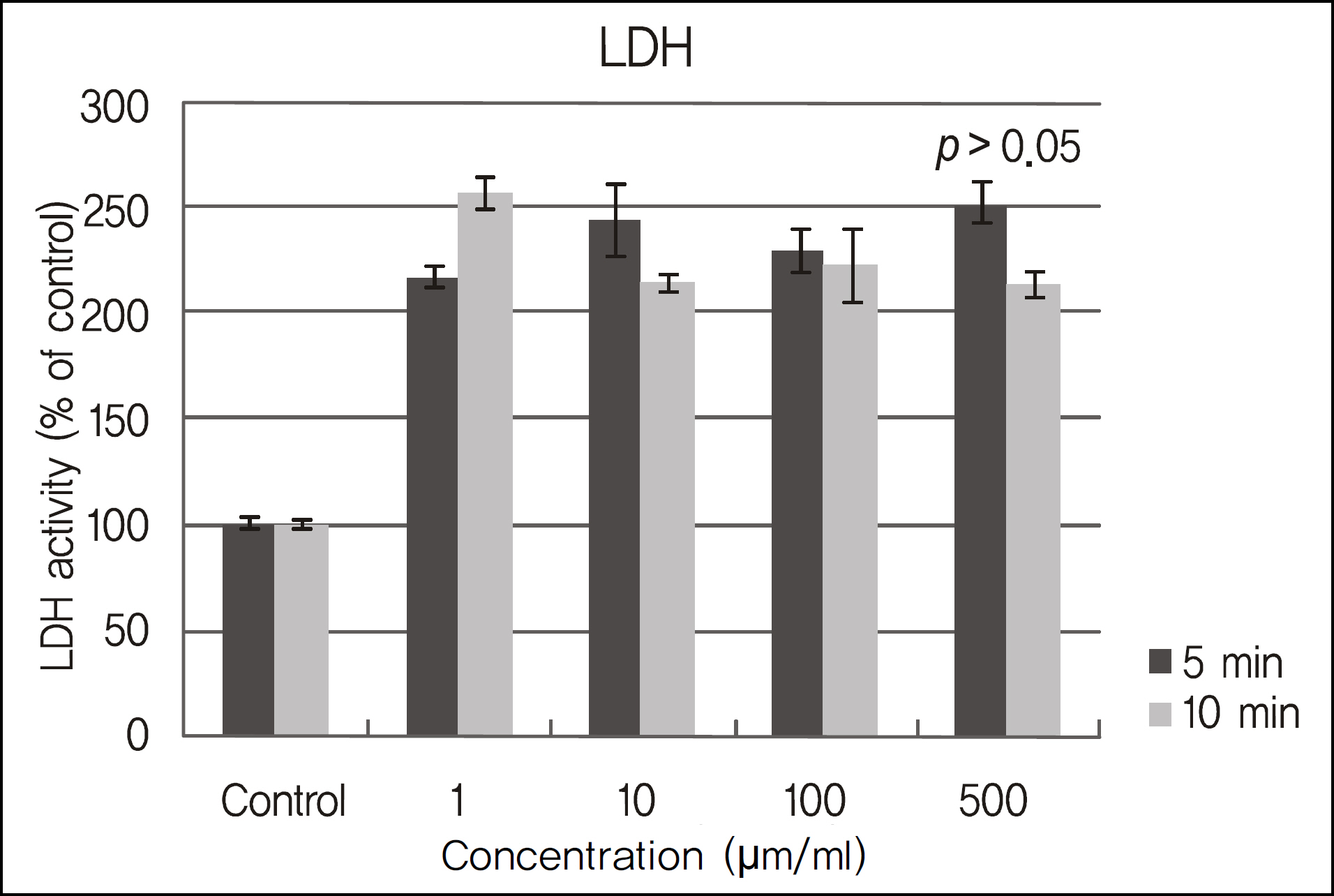
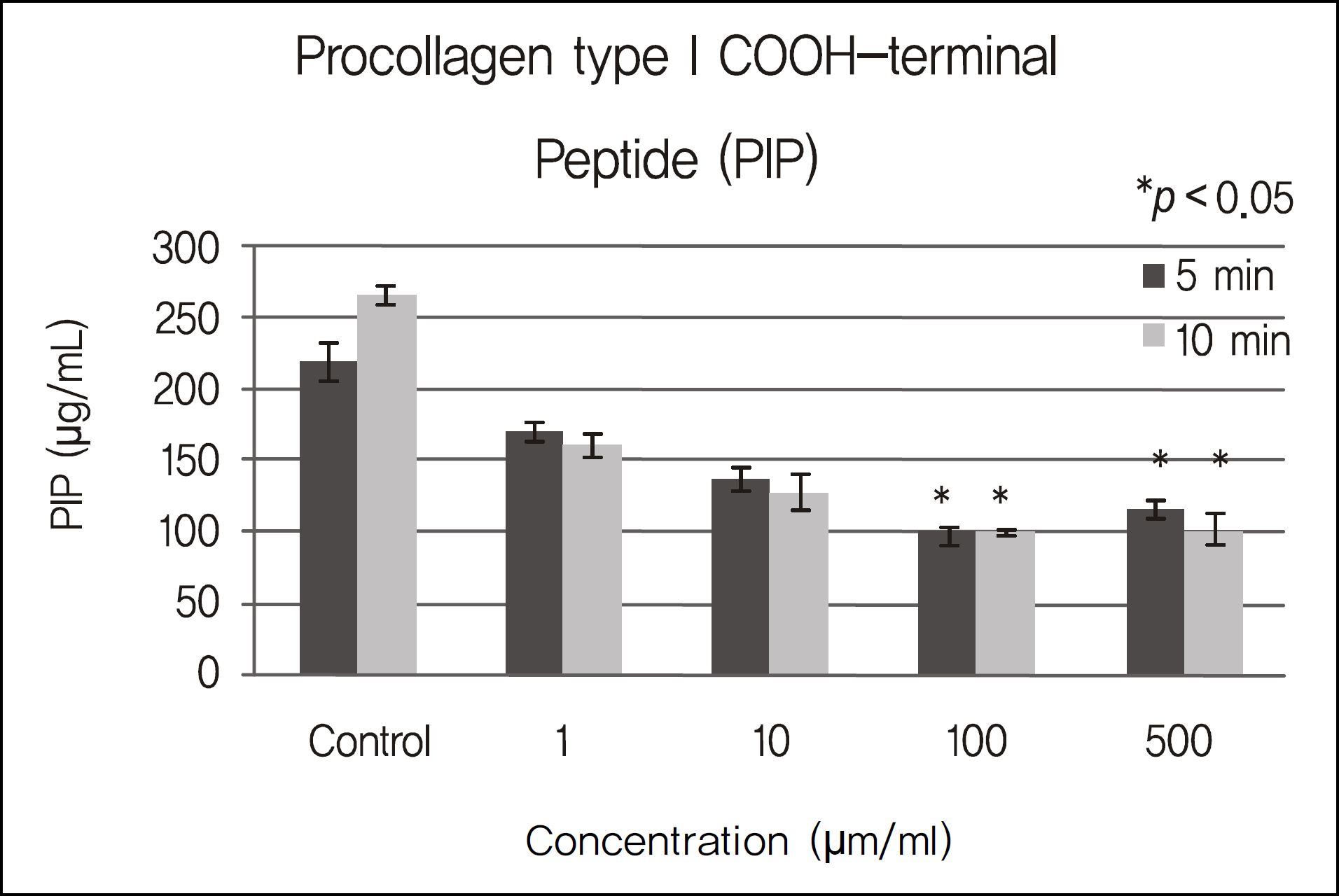
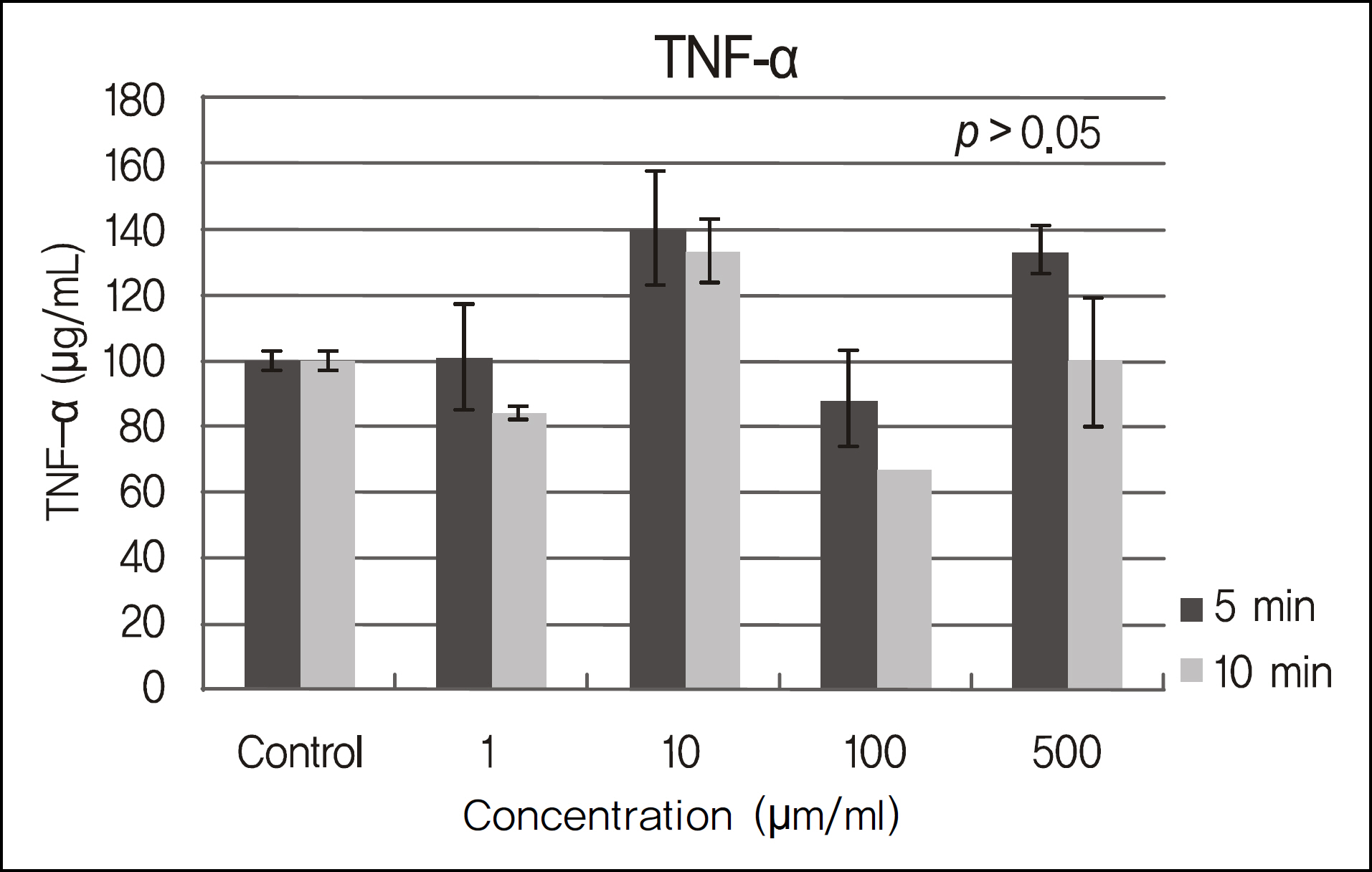
The inhibitory effects of human corneal epithelial cellular proliferation did not show a concentration- or exposure time-dependent response. Activity of LDH did not show a statistically significant difference for the different concentrations and exposure times. The inhibitory effects of extracellular matrix proteins such as PIP or laminin synthesized from human corneal epithelial cells were compared with those in the control group and showed a statistically significant difference at a cyclosporine concentration greater than 0.01%. Released TNF-alpha and IL-1 from human corneal epithelial cells did not show any difference according to concentration or cyclosporine exposure time.
CONCLUSIONS
To modulate human corneal epithelial cellular proliferation and the levels of extracellular matrix proteins such as PIP and laminin, a concentration of cyclosporine A greater than 0.01% for longer than 5 minutes of exposure is needed. There were little effects of cyclosporine A on pro-inflammatory cytokine secreted from corneal epithelial cells.
Keyword
MeSH Terms
-
Cell Proliferation
Cyclosporine
Enzyme-Linked Immunosorbent Assay
Epithelial Cells
Epithelium, Corneal
Extracellular Matrix Proteins
Humans
Interleukin-1
L-Lactate Dehydrogenase
Laminin
Tumor Necrosis Factor-alpha
Cyclosporine
Extracellular Matrix Proteins
Interleukin-1
L-Lactate Dehydrogenase
Laminin
Tumor Necrosis Factor-alpha
Figure
Reference
-
References
1. Hill JC. Immunosuppression in corneal transplantation. Eye. 1995; 9:247–53.
Article2. Dick AD, Azim M, Forrester JV. Immunosuppressive therapy for chronic uveitis: optimising therapy with steroids and cyclosporine A. Br J Ophthalmol. 1997; 81:1107–12.3. Masuda K, Nakajima A, Urayama A, et al. Double-masked trial of cyclosporin versus colchicine and long-term open study of cyclosporin in Behcet's disease. Lancet. 1989; 1:1093–6.4. Le Hoang P, Girard B, Deray G, et al. Cyclosporin A in the treatment of birdshot retinochoroidopathy. Transplant Proc. 1988; 20:128–30.5. Stepkowski SM. Molecular targets for existing and novel immunosuppressive drugs. Expert Rev Mol Med. 2000; 2:1–23.
Article6. Minguez E, Tiestos MT, Cristobal JA, et al. Intraocular absorption of cyclosporin A eyedrops. J Fr Ophthalmol. 1992; 15:263–7.7. Oh C, Apel AJ, Saville BA, et al. Local efficacy of cyclosporine in corneal transplant therapy. Curr Eye Res. 1994; 13:337–43.8. BenEzra D, Matamoros N, Cohen E. Treatment of severe vernal keratoconjunctivitis with cyclosporine A eyedrops. Transplant Proc. 1988; 20:644–9.9. Calonge M. The treatment of dry eye. Surv Opthahlmol. 2001; 45(suppl. 2):S227–39.10. Díaz-Valle D, Benítez del Castillo JM, Castillo A, et al. Immunologic and clinical evaluation of postsurgical necrotizing sclerocorneal ulceration. Cornea. 1998; 17:371–5.
Article11. Sall K, Stevenson OD, Mundorf TK, Reis BL. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology. 2000; 107:631–9.12. Nussenblatt RB, Palestine AG. Cyclosporin: immunology, pharmacology and therapeutic uses. Surv Ophthalmol. 1986; 31:159–69.13. Power WJ, Mullaney P, Farrel M, et al. Effect of topical cyclosporin A on conjunctival T cells in patients with secondary Sjögren's syndrome. Cornea. 1993; 12:507–11.
Article14. Donnenfeld E, Pflugfelder SC. Topical ophthalmic cyclosporine: pharmacology and clinical uses. Surv Ophthalmol. 2009; 54:321–38.15. Tatlipinar S, Akpek EK. Topical ciclosporin in the treatment of ocular surface disorder. Br J Ophthalmol. 2005; 89:1363–7.16. Wiederholt M, Kössendrup D, Schulz W, Hoffmann F. Pharmacokinetic of topical cyclosporin A in the rabbit eye. Invest Ophthalmol Vis Sci. 1986; 27:519–24.17. Acheampong AA, Shcakleton M, Tang-Liu DD, et al. Distrubution of cyclosporin A in ocular tissues after topical administration to albino rabbits and beagle dogs. Curr Eye Res. 1999; 18:91–103.18. Lee LE, Shin WB, Lee JS. Effect of cyclosporine A 0.05% on human corneal epithelial cells. J Korean Ophthalmol. 2007; 48:1399–1409.
Article19. Garweg JG, Wegmann-Burns M, Goldblum D. Effects of daunor-ubicin, mitomycin C, azathiprine and cyclosporin A on human retinal pigmented epithelial, corneal endothelial and conjunctival cell lines. Graefes Arch Clin Exp Ophthalmol. 2006; 244:382–9.20. Mauger TF, Craig EL. Havener's Ocular Pharmacology. 6th ed.St. Lousi: Mosby;1994. p. 28.21. Singh G, Lindstrom RL, Doughman DJ. Cyclosporin A on human corneal endothelium. Cornea. 1984; 3:272–7.
Article22. BenEzra D, Antebe I, Maftzir G. Differential effect of cyclosporin A on lymphocyte and keratocyte proliferation. Invest Ophthalmol Vis Sci. 1987; 28:42.23. Tonoe O, Yamanaka O, Okada Y, et al. Collagen biosynthesis and cellular proliferation after filtering surgery in rabbits. Folia Ophthalmol Jpn. 1995; 46:242–5.24. Berman B, Duncan MR. Pentoxifylline inhibits normal hyman dermal fibroblast in vitro proliferation, collagen, glycosaminoglycan, and fibronectin production, and increases collagenase activity. J Invest Dermatol. 1989; 92:605–10.25. Leonardi A, DeFranchis G, Fregona IA, et al. Effects of cyclosporine A on human conjunctival fibroblasts. Arch Ophthalmol. 2001; 119:1512–7.26. Bankers-Fulbright JL, Kalli KR, McKean DJ. Interleukin-1 signal transduction. Life Sciences. 1996; 59:61–83.
Article27. Li DQ, Lokeshwar BL, Solomon A, et al. Regulation of MMP-9 production by human corneal epithelial cells. Exp Eye Res. 2001; 73:449–59.
Article28. Bargagna-Mohan P, Strissel KJ, Fini ME. Regulation of gelatinase B production in corneal cells is independent of autocrine IL-1alpha. Invest Ophthalmol Vis Sci. 1999; 40:784–9.29. Djalilian AR, Nagineni CN, Mahesh SP, et al. Inhibition of inflammatory cytokine production in human corneal cells by dexamethasone, but not cyclosporin. Cornea. 2006; 25:709–14.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Cyclosporine A 0.05% on Human Corneal Epithelial Cells
- Expression of Local Immunosuppressive Factor, Indoleamine 2,3-dixygenase, in Human Coreal Cells
- Effects of Damaged Human Corneal Epithelial Cells on Differentiation of Human Mesenchymal Stem Cell
- Effects of Human Serum on Human Corneal Epithelial Cells in Vitro
- Long-term Outcome of Limbal Epithelial Cells Cultivated in Vivo on Amniotic Membrane Transplantation