J Korean Soc Clin Pharmacol Ther.
2012 Jun;20(1):42-50.
Comparison of Pharmacokinetic Characteristics and the Safety between Amlodipine Maleate Tablet 5 mg and Amlodipine Besylate Tablet 5 mg
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, Seoul, Korea. ksbae@amc.seoul.kr
- 2University of Ulsan College of Medicine, Korea.
- 3School of Military Medicine, The First Republic of Korea Army, Korea.
- 4Department of Clinical Pharmacology and Therapeutics, Inje University Pusan Paik Hospital, Pusan, Korea.
- 5Division of Clinical Pharmacology, Clinical Trials Center, Pusan National University Hospital, Korea.
- 6Division of Clinical Pharmacology, Clinical Trial Center, Samsung Medical Center, Korea.
- 7Technical research center, CKD research institute, Chong Kun Dang Pharmaceutical Co, Ltd, Korea.
Abstract
- BACKGROUND
Amlodipine is a third-generation dihydropyridine calcium channel blocker for treating hypertension. Though marketed primarily as a besylate salt, there have been some efforts to find other comparable salts. Among them, maleate is the salt that has been considered favorable for many drugs. The aim of this study was to compare the pharmacokinetics, as well as safety and tolerability of amlodipine maleate with amlodipine besylate.
METHODS
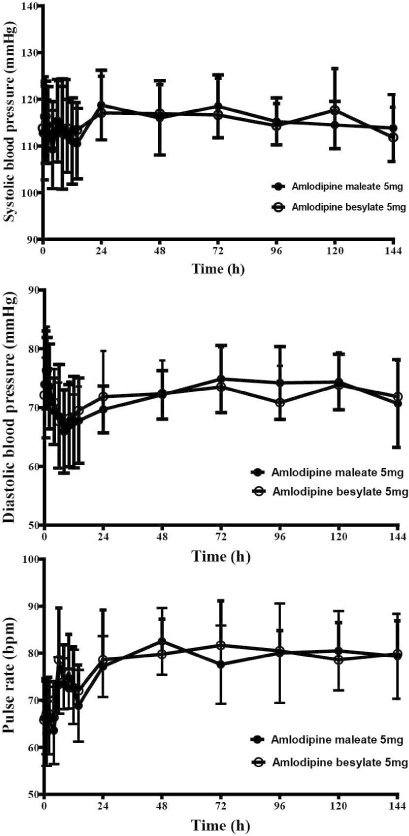
This study was open, randomized, two-period crossover design investigated in twelve healthy male volunteers over a 144 h period after administrating two forms of amlodipine 5 mg, respectively. Each period was separated with 2 weeks. Plasma concentrations of amlodipine were determined by liquid chromatography-tandem mass spectrometry. Safety profiles were assessed by vital signs, physical examinations, electrocardiograms, laboratory testing and adverse events monitoring.
RESULTS
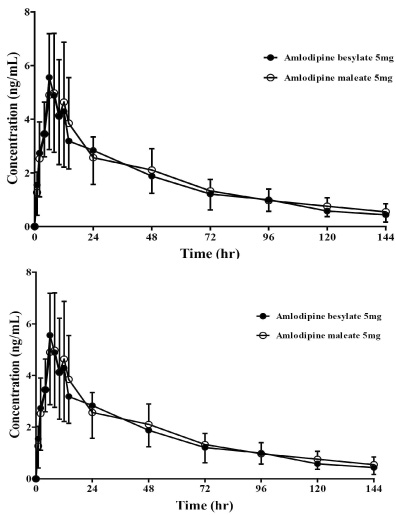
All subjects were completed this study. Geometric mean ratios (GMRs) of amlodipine maleate/amlodipine besylate of Cmax and AUClast for amlodipine were 0.92 (90 % confidence interval, 0.81 ~ 1.05) and 1.05 (0.96 ~ 1.16), respectively. No serious adverse events were reported, and no clinically relevant changes were observed in safety profiles during this trial.
CONCLUSION
Pharmacokinetics, tolerability and the safety were comparable between amlodipine maleate and amlodipine besylate in healthy individuals.
MeSH Terms
Figure
Reference
-
1. Benetos A, Consoli S, Safavian A, Dubanchet A, Safar M. Efficacy, safety, and effects on quality of life of bisoprolol/hydrochlorothiazide versus amlodipine in elderly patients with systolic hypertension. Am Heart J. 2000. 140(4):E11.
Article2. Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005. 365(9455):217–223.
Article3. Franco OH, Peeters A, Bonneux L, de Laet C. Blood pressure in adulthood and life expectancy with cardiovascular disease in men and women: life course analysis. Hypertension. 2005. 46(2):280–286.
Article4. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003. 289(19):2560–2572.
Article5. Mason RP, Marche P, Hintze TH. Novel vascular biology of third-generation L-type calcium channel antagonists: ancillary actions of amlodipine. Arterioscler Thromb Vasc Biol. 2003. 23(12):2155–2163.
Article6. Beresford AP, McGibney D, Humphrey MJ, Macrae PV, Stopher DA. Metabolism and kinetics of amlodipine in man. Xenobiotica. 1988. 18(2):245–254.
Article7. Hansson L, Lindholm LH, Ekbom T, Dahlof B, Lanke J, Schersten B, Wester PO, Hedner T, de Faire U. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 1999. 354(9192):1751–1756.
Article8. Burges R, Moisey D. Unique pharmacologic properties of amlodipine. Am J Cardiol. 1994. 73(3):2A–9A.
Article9. van Zwieten PA. Amlodipine: an overview of its pharmacodynamic and pharmacokinetic properties. Clin Cardiol. 1994. 17:9 Suppl 3. III3–III6.10. Park JY, Kim KA, Lee GS, Park PW, Kim SL, Lee YS, Lee YW, Shin EK. Randomized, open-label, two-period crossover comparison of the pharmacokinetic and pharmacodynamic properties of two amlodipine formulations in healthy adult male Korean subjects. Clin Ther. 2004. 26(5):715–723.
Article11. New amlodipine (Amloc) product available in South Africa. Cardiovasc J S Afr. 2005. 16(1):61.12. Lee HY, Kang HJ, Koo BK, Oh BH, Heung-Sun K, Kim KS, Seo HS, RO YM, Kang JH, Woong CJ, Joo SJ, Kim MH, Joon-Han S, Yoon J, Park SH, Jin-Ok J, Ju AK, Chong-Yun R, Yeon KJ, Park KM, Lim DK, Park SY. Clinic blood pressure responses to two amlodipine salt formulations, adipate and besylate, in adult Korean patients with mild to moderate hypertension: a multicenter, randomized, double-blind, parallel-group, 8-week comparison. Clin Ther. 2005. 27(6):728–739.
Article13. Park S, Chung N, Kwon J, Yoon JH, Kim YJ, Han DS, Kim HS. Results of a multicenter, 8-week, parallel-group, randomized,double-blind, double-dummy, Phase III clinical trial to evaluate the efficacy and tolerability of amlodipine maleate versus amlodipine besylate in Korean patients with mild to moderate hypertension. Clin Ther. 2005. 27(4):441–450.
Article14. Carvalho M, Oliveira CH, Mendes GD, Sucupira M, Moraes ME, De Nucci G. Amlodipine bioequivalence study: quantification by liquid chromatography coupled to tandem mass spectrometry. Biopharm Drug Dispos. 2001. 22(9):383–390.
Article15. Hong SJ, Ahn TH, Baek SH, Cho WH, Jeon HK, Kwan J, Yoon MH, Lee KJ, Lim DS. Comparison of efficacy and tolerability of amlodipine orotate versus amlodipine besylate in adult patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group, 8-week follow-up, noninferiority trial. Clin Ther. 2006. 28(4):537–551.
Article16. Kes S, Caglar N, Canberk A, Deger N, Demirtas M, Dortlemez H, Kiliccioglu B, Kozan O, Ovunc K, Turkoglu C. Treatment of mild-to-moderate hypertension with calcium channel blockers: a multicentre comparison of once-daily nifedipine GITS with once-daily amlodipine. Curr Med Res Opin. 2003. 19(3):226–237.
Article17. Meredith PA. Potential concerns about generic substitution: bioequivalence versus therapeutic equivalence of different amlodipine salt forms. Curr Med Res Opin. 2009. 25(9):2179–2189.
Article18. Mignini F, Tomassoni D, Traini E, Amenta F. Single-dose, randomized, crossover bioequivalence study of amlodipine maleate versus amlodipine besylate in healthy volunteers. Clin Exp Hypertens. 2007. 29(8):539–552.
Article19. Jeon JY, Kim JR, Chung JY, Oh DS, Cho JY, Yu KS, Jang IJ, Shin SG. Pharmacokinetic and pharmacodynamic comparative study of amlodipine adipate and amlodipine besylate. J Korean Soc Clin Pharmacol Ther. 2006. 14(2):106–115.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Pharmacokinetic Characteristics and Safety Between JW Amlodipine(R) Tablet 5 mg and Novarsc(R) Tablet 5 mg in Healthy Male Volunteers
- Efficacy and Safety of Amlodipine Camsylate(Amodipin(TM)) for Treatment of Essential Hypertension
- Amlodipine toxicity complicated by concurrent medications
- Is irrational use of intralipid emulsion justified in amlodipine toxicity?
- Pharmacokinetic comparison between a fixed-dose combination of fimasartan/amlodipine/ hydrochlorothiazide 60/10/25 mg and a corresponding loose combination of fimasartan/amlodipine 60/25 mg and hydrochlorothiazide 25 mg in healthy subjects