J Korean Soc Clin Pharmacol Ther.
2013 Dec;21(2):95-103. 10.12793/jkscpt.2013.21.2.95.
Comparison of Pharmacokinetic Characteristics and Safety Between JW Amlodipine(R) Tablet 5 mg and Novarsc(R) Tablet 5 mg in Healthy Male Volunteers
- Affiliations
-
- 1Department of Clinical Pharmacology and Therapeutics, Asan Medical Center, Seoul, Korea. ksbae@amc.seoul.kr
- 2Department of Clinical Pharmacology and Therapeutics, University of Ulsan College of Medicine, Korea.
- 3Department of Clinical Pharmacology, Inha University Hospital, Inha University Scull Of Medicine, Incheon, Korea.
- 4Department of Clinical Pharmacology, Busan Paik Hospital, Busan, Korea.
- 5Clinical Trials Center, Pusan National University Hospital, Busan, Korea.
- 6Clinical Trial Center, Samsung Medical Center, Seoul, Korea.
- KMID: 2203222
- DOI: http://doi.org/10.12793/jkscpt.2013.21.2.95
Abstract
- BACKGROUND
Amlodipine is a third-generation dihydropyridine calcium channel blocker, which has proven to be a useful drug against hypertension or angina.
METHODS
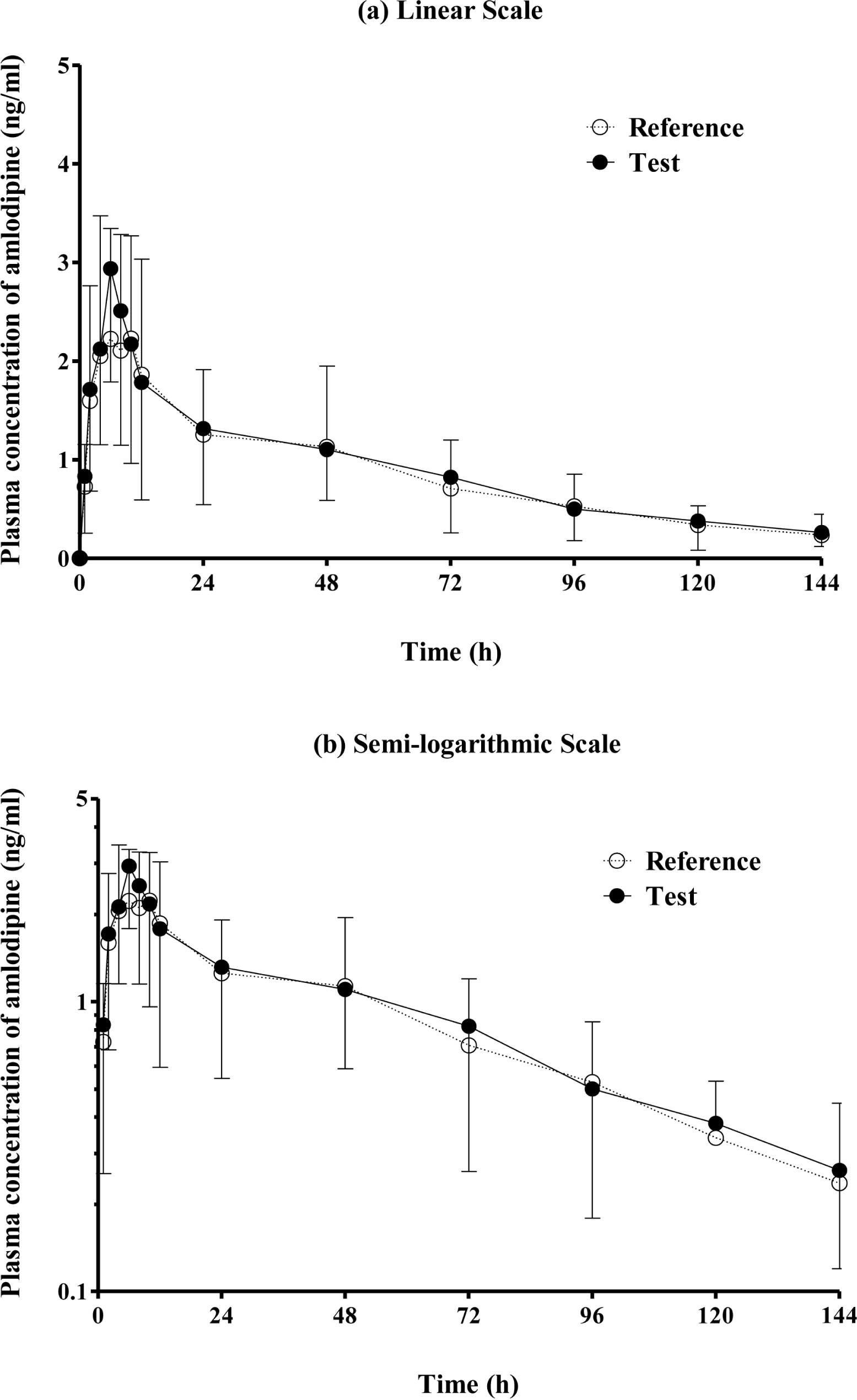
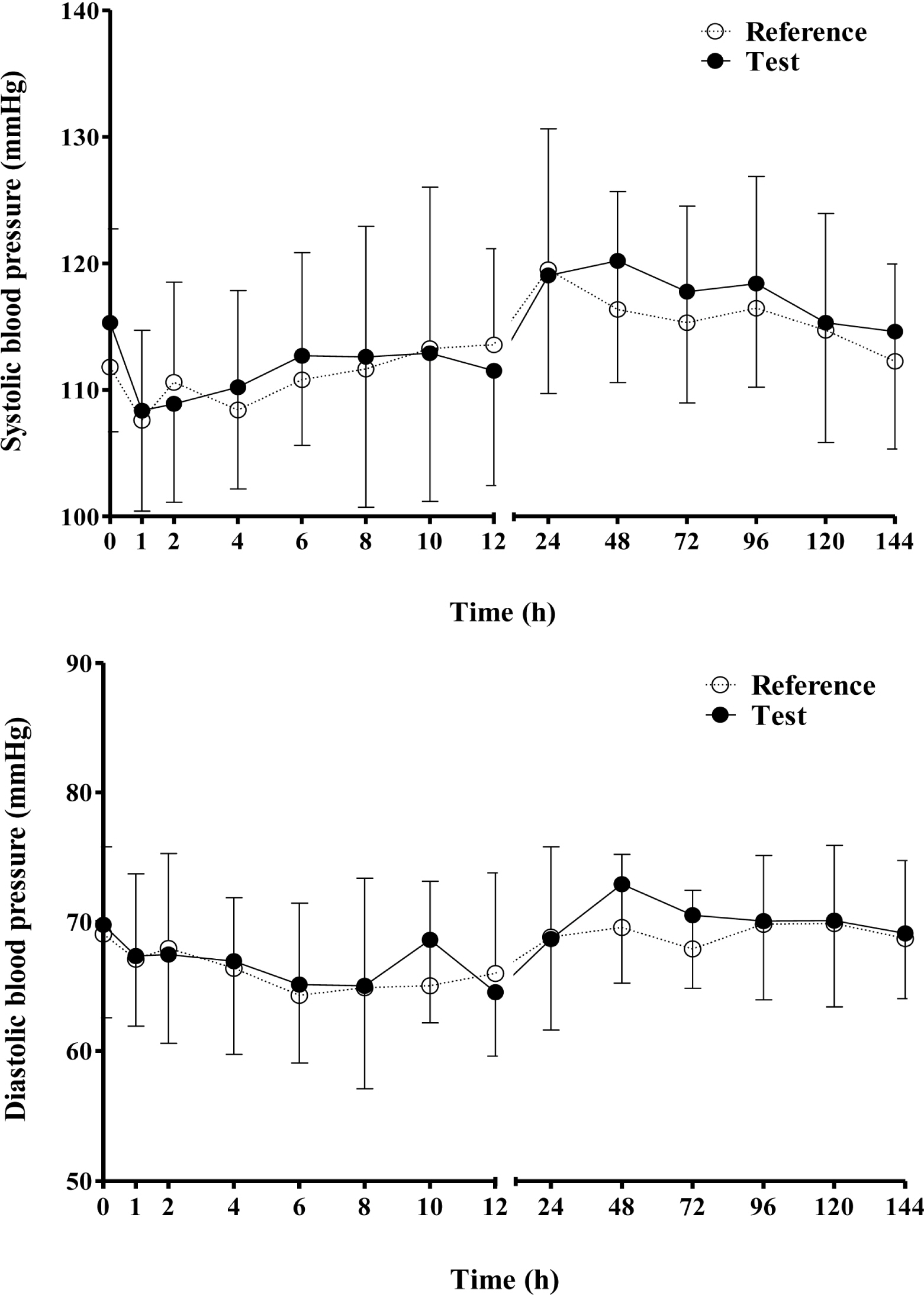
This randomized, open-label, two-period, two-treatment, single-dose, crossover study was conducted in twenty healthy male volunteers. Subjects were administered 5 mg of the test or reference formulation. After 2-week washout period, the other formulation was administered. Blood samples were collected up to 144 hours after drug administration, and plasma amlodipine concentrations were determined by validated liquid chromatography-tandem mass spectrometry. Drug safety was assessed using measurement of vital signs, physical examinations, laboratory test, electrocardiograms, and adverse event monitoring.
RESULTS
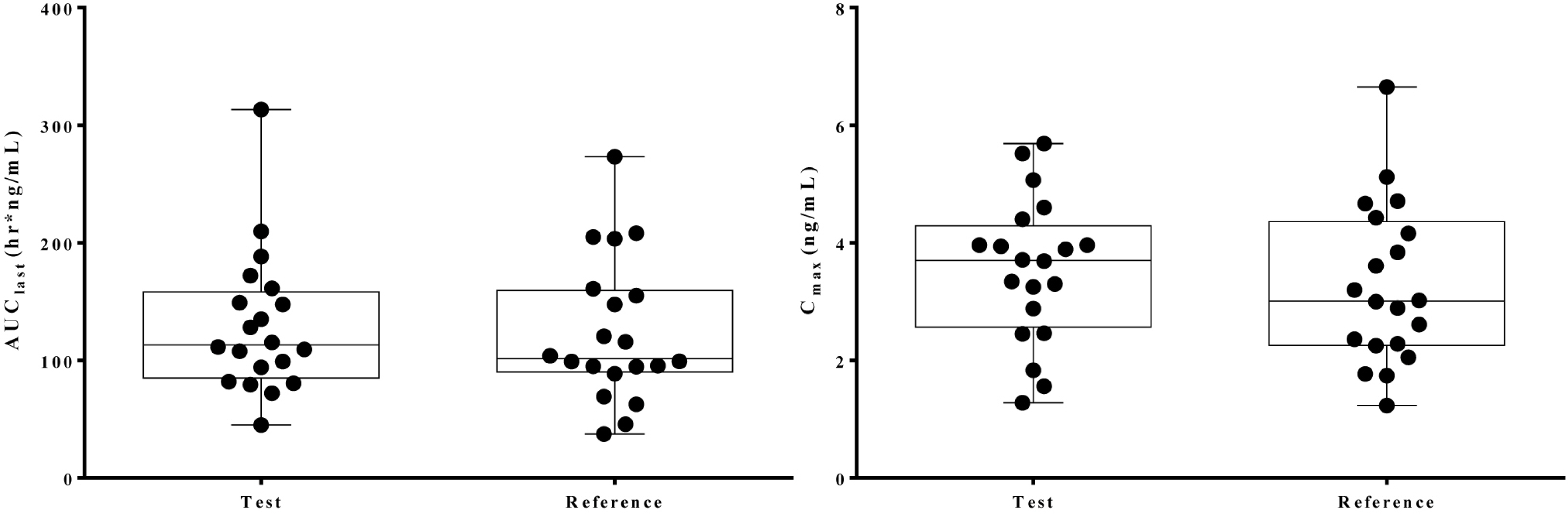
All subjects were completed this study. The geometric mean ratios of Cmax and AUClast were 1.078 (90 % CI, 0.968 - 1.200) and 1.095 (90 % CI, 1.011 - 1.186), respectively. There were no serious adverse events were reported by both formulations.
CONCLUSION
This study showed the test and reference formulations had similar pharmacokinetics and safety profiles.
Keyword
MeSH Terms
Figure
Reference
-
1. Abernethy DR, Schwartz JB. Calcium-antagonist drugs. N Engl J Med. 1999; 341(19):1447–1457.
Article2. Opie LH, Yusuf S, Kubler W. Current status of safety and efficacy of calcium channel blockers in cardiovascular diseases: a critical analysis based on 100 studies. Prog Cardiovasc Dis. 2000; 43(2):171–196.
Article3. Mason RP, Marche P, Hintze TH. Novel vascular biology of third-generation L-type calcium channel antagonists: ancillary actions of amlodipine. Arterioscler Thromb Vasc Biol. 2003; 23(12):2155–2163.4. Spedding M, Paoletti R. Classification of calcium channels and the sites of action of drugs modifying channel function. Pharmacol Rev. 1992; 44(3):363–376.5. Meredith PA, Elliott HL. Clinical pharmacokinetics of amlodipine. Clin Phamacokinet. 1992; 22(1):22–31.
Article6. Haria M, Wagstaff AJ. Amlodipine. A reappraisal of its pharmacological properties and therapeutic use in cardiovascular disease. Drugs. 1995; 50(3):560–586.7. Minami J, Ishimitsu T, Kawano Y, Matsuoka H. Effects of amlodipine and nifedipine retard on autonomic nerve activity in hypertensive patients. Clin Exp Pharmacol Physiol. 1998; 25(7-8):572–576.
Article8. Hosie J, Bremner AD, Fell PJ, James IG, Saul PA, Taylor SH. Comparison of early side effects with amlodipine and nifedipine retard in hypertension. Cardiology. 1992; 80(Suppl 1):54–59.
Article9. Lee HY, Kang HJ, Koo BK, Oh BH, Heung-Sun K, Kim KS, Seo HS, Ro YM, Kang JH, Woong CJ, Joo SJ, Kim MH, Joon-Han S, Yoon J, Park SH, Jin-Ok J, Ju AK, Chong-Yun R, Yeon KJ, Park KM, Lim DK, Park SY, Amostar Study I. Clinic blood pressure responses to two amlodipine salt formulations, adipate and besylate, in adult Korean patients with mild to moderate hypertension: a multicenter, randomized, double-blind, parallel-group, 8-week comparison. Clin Ther. 2005; 27(6):728–739.
Article10. Hong SJ, Ahn TH, Baek SH, Cho WH, Jeon HK, Kwan J, Yoon MH, Lee KJ, Lim DS. Comparison of efficacy and tolerability of amlodipine orotate versus amlodipine besylate in adult patients with mild to moderate hypertension: a multicenter, randomized, double-blind, placebo-controlled, parallel-group, 8-week follow-up, noninferiority trial. Clin Ther. 2006; 28(4):537–551.
Article11. Kim SH, Kim YD, Lim DS, Yoon MH, Ahn YK, On YK, Lee JW, Kim IJ, Park JB, Kim JJ, Chung WS, Yang JY, Seo HS, Shin EK, Kim HS; Korean Multicenter Amlodipine Study I. Results of a phase III, 8-week, multicenter, prospective, randomized, double-blind, parallel-group clinical trial to assess the effects of amlodipine camsylate versus amlodipine besylate in Korean adults with mild to moderate hypertension. Clin Ther. 2007; 29(9):1924–1936.
Article12. Kim SA, Park S, Chung N, Lim DS, Yang JY, Oh BH, Tahk SJ, Ahn TH. Efficacy and safety profiles of a new S(-)-amlodipine nicotinate formulation versus racemic amlodipine besylate in adult Korean patients with mild to moderate hypertension: an 8-week, multicenter, randomized, double-blind, double-dummy, parallel-group, phase III, noninferiority clinical trial. Clin Ther. 2008; 30(5):845–857.
Article13. Park JY, Kim KA, Park PW, Lee OJ, Ryu JH, Lee GH, Ha MC, Kim JS, Kang SW, Lee KR. Pharmacokinetic and pharmacodynamic characteristics of a new S-amlodipine formulation in healthy Korean male subjects: a randomized, open-label, two-period, comparative, crossover study. Clin Ther. 2006; 28(11):1837–1847.
Article14. Faulkner JK, McGibney D, Chasseaud LF, Perry JL, Taylor IW. The pharmacokinetics of amlodipine in healthy volunteers after single intravenous and oral doses and after 14 repeated oral doses given once daily. Br J Clin Pharmacol. 1986; 22(1):21–25.
Article15. Josefsson M, Zackrisson AL, Ahlner J. Effect of grapefruit juice on the pharmacokinetics of amlodipine in healthy volunteers. Eur J Clin Pharmacol. 1996; 51(2):189–193.
Article16. Williams DM, Cubeddu LX. Amlodipine pharmacokinetics in healthy volunteers. J Clin Pharmacol. 1988; 28(11):990–994.
Article17. Farolfi M, Powers JD, Rescigno A. On the determination of bioequivalence. Pharmacol Res. 1999; 39(1):1–4.
Article18. Park S, Chung N, Kwon J, Yoon JH, Kim YJ, Han DS, Kim HS. Results of a multicenter, 8-week, parallel-group, randomized, double-blind, double-dummy, Phase III clinical trial to evaluate the efficacy and tolerability of amlodipine maleate versus amlodipine besylate in Korean patients with mild to moderate hypertension. Clin Ther. 2005; 27(4):441–450.19. Marzo A. Open questions on bioequivalence: some problems and some solutions. Pharmacol Res. 1999; 40(4):357–368.
Article20. Ramirez E, Laosa O, Guerra P, Duque B, Mosquera B, Borobia AM, Lei SH, Carcas AJ, Frias J. Acceptability and characteristics of 124 human bioequivalence studies with active substances classified according to the Biopharmaceutic Classification System. Br J Clin Pharmacol. 2010; 70(5):694–702.
Article21. Abad-Santos F, Novalbos J, Galvez-Mugica MA, Gallego-Sandin S, Almeida S, Vallee F, Garcia AG. Assessment of sex differences in pharmacokinetics and pharmacodynamics of amlodipine in a bioequivalence study. Pharmacol Res. 2005; 51(5):445–452.
Article22. Park JY, Kim KA, Lee GS, Park PW, Kim SL, Lee YS, Lee YW, Shin EK. Randomized, open-label, two-period crossover comparison of the pharmacokinetic and pharmacodynamic properties of two amlodipine formulations in healthy adult male Korean subjects. Clin Ther. 2004; 26(5):715–723.
Article23. Valcarcel Y, Jimenez R, Aristegui R, Gil A, Nota Study G. Effectiveness and Safety of Amlodipine in Newly Diagnosed Hypertensive Patients and in Previously Diagnosed Hypertensive Patients not Controlled with their Usual Treatment (NOTA Study). Clin Drug Investig. 2003; 23(12):761–770.24. Yi S, Kim TE, Yoon SH, Cho JY, Shin SG, Jang IJ, Yu KS. Pharmacokinetic interaction of fimasartan, a new angiotensin II receptor antagonist, with amlodipine in healthy volunteers. J Cardiovasc Pharmacol. 2011; 57(6):682–689.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Pharmacokinetic Characteristics and the Safety between Amlodipine Maleate Tablet 5 mg and Amlodipine Besylate Tablet 5 mg
- Pharmacokinetic Equivalence of the High Dose Strength Fixed-Dose Combination Tablet of Gemigliptin/Metformin Sustained Release (SR) and Individual Component Gemigliptin and Metformin XR Tablets in Healthy Subjects
- Pharmacokinetics and Safety of Levodropropizine Controlled Release Tablet after Repeated Dosing in Healthy Male Volunteers
- Bioequivalence and Dose Proportionality of Olmesartan Medoxomil Formulations
- Bioequivalence study of Donepezil hydrochloride in healthy Korean volunteers