J Korean Assoc Oral Maxillofac Surg.
2010 Oct;36(5):353-359.
The immunosuppression effect of cyclosporine A on the allogenic calvarial bone graft in mice
- Affiliations
-
- 1Department of Oral and Maxillofacial Surgery, School of Dentistry, Chonnam National University, Gwangju, Korea. hkoh@jnu.ac.kr
Abstract
- INTRODUCTION
This study examined the effect of cyclosporine A (CsA) on the allogenic cranial bone graft in the mice.
MATERIALS AND METHODS
Twenty eight 12-week-old male ICR mice weighing 40 g were used. The experimental group was injected subcutaneously with CsA (10 mg/kg/day) diluted in Caster oil for 7 days prior to the graft until sacrifice. The control group was injected with the same solution without CsA.
RESULTS
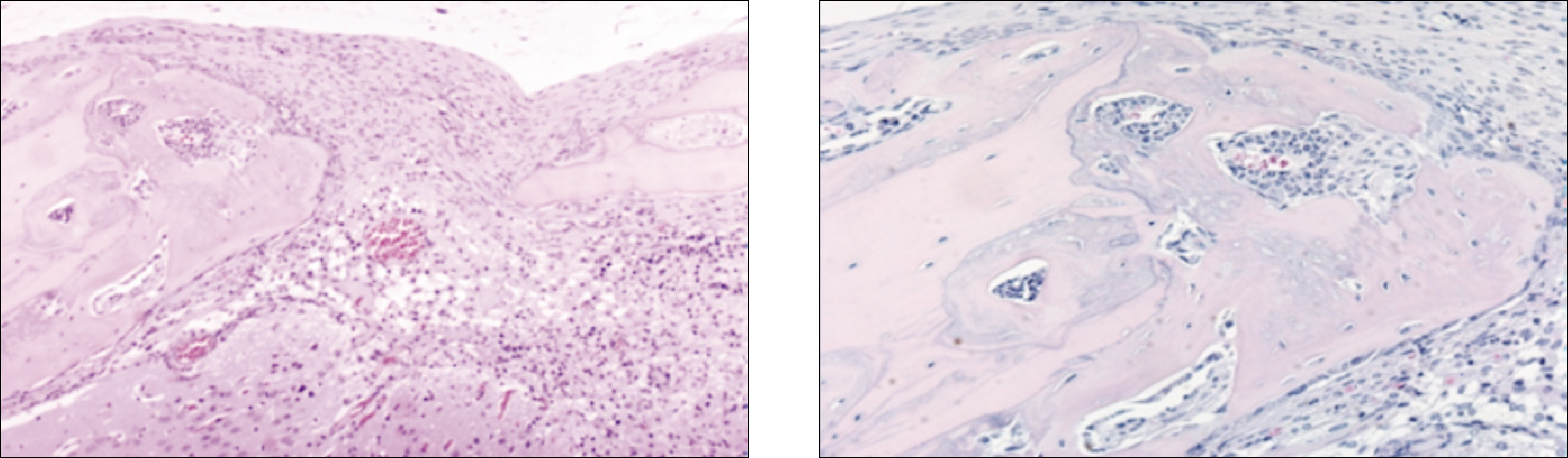
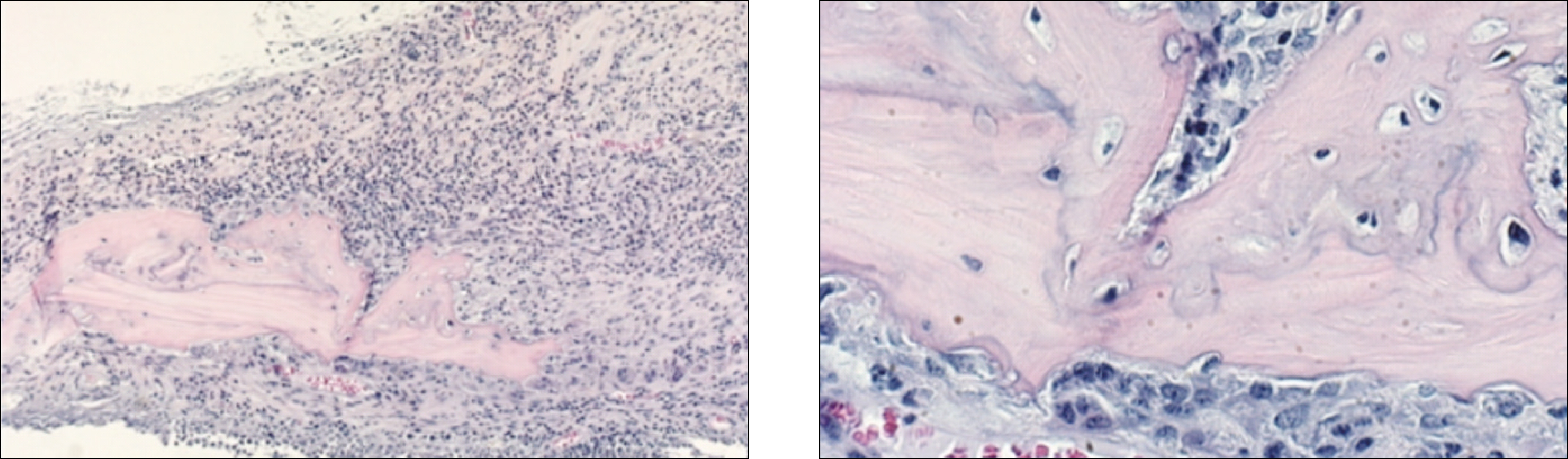
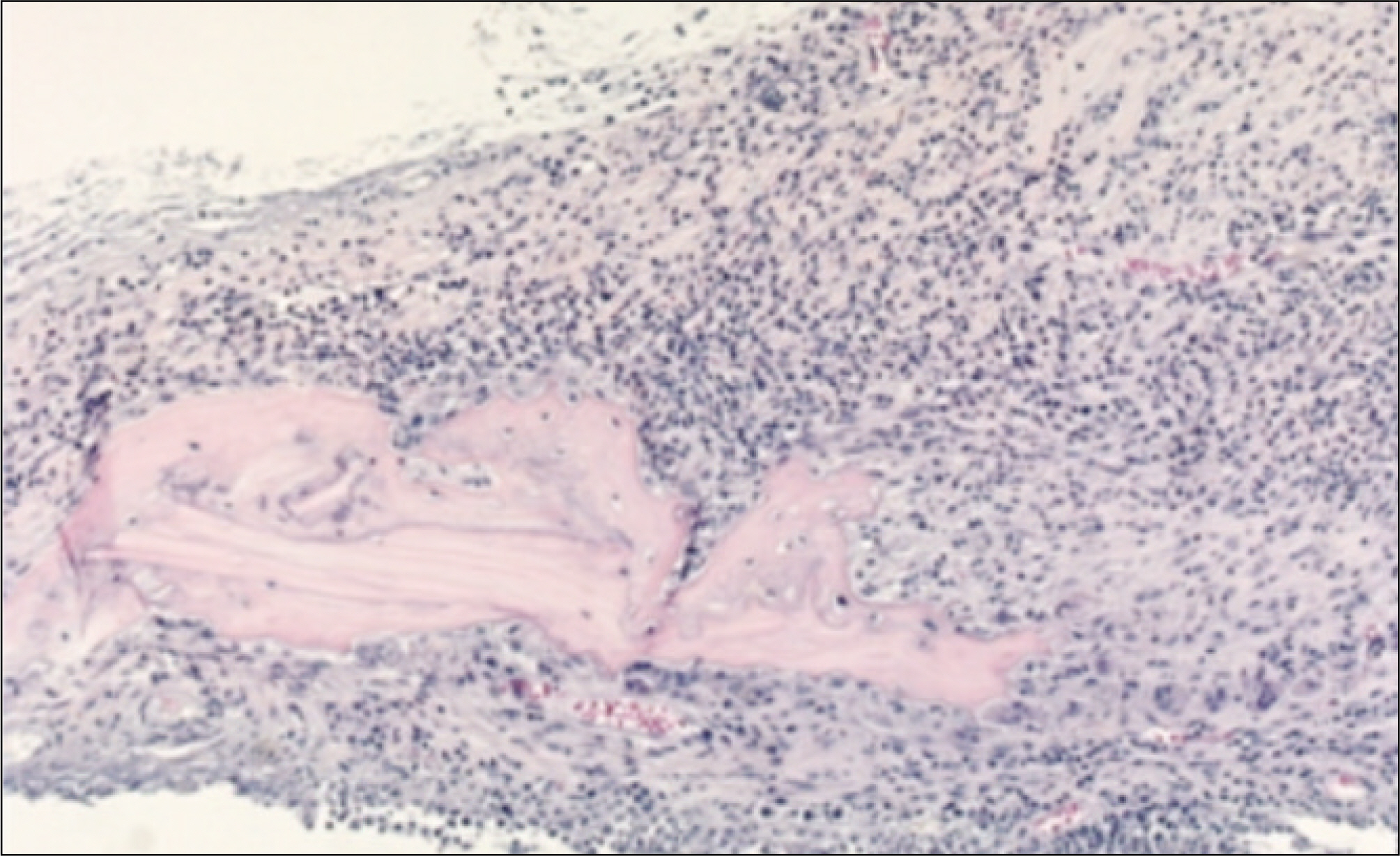
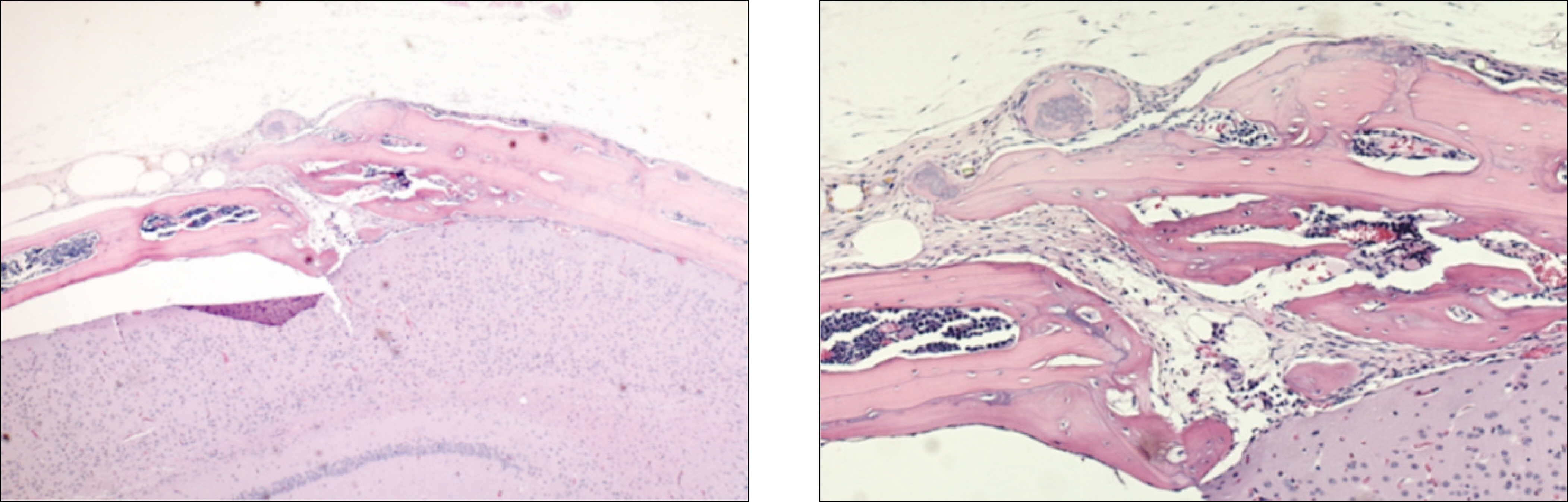
In the experimental group, fibrous connective tissues and small amounts of inflammatory cells were observed. At 2 weeks after the allograft in the experimental group, new bone formation in fibrous collagenous tissue and around the allogenic bone was noted. At 4 weeks after the allograft, new bone formation was active along and at the periphery of the mature allogenic bone. The proliferation of blood vessels increased in bone marrow. In the control group, fibrous tissues and inflammatory cells were observed around the allogenic bone and existing bone at 1 week. At 2 weeks after the allograft, the proliferation of blood vessels accompanied by inflammatory cells were scattered in the fibrous connective tissues. New bone formation around the allogenic and existing bone could be observed. At 4 weeks after the allograft, inflammatory cells were severely infiltrated around the allogenic bone. Osteoclasts were scattered along the allogenic bone and induced bone resorption.
CONCLUSION
These results suggest that the daily administration of CsA (10 mg/kg/day) induces efficient immunosuppression without serious complications ,and this protocol might be useful for the experimental model of allogenic bone grafts.
MeSH Terms
Figure
Reference
-
References
1. Yanagihara RH, Adler WH. Inhibition of mouse natural killer activity by cyclosporin A. Immunology. 1982; 45:325–32.2. Wachowiak J. Effects of cyclosporin A on the activity of mouse natural killer cells and hybrid resistance. Immunol Lett. 1986; 13:95–9.
Article3. Yanagihara RH, Adler WH. Cyclosporin a inhibits T cell-mediated augmentation of mouse natural killer activity. Immunopharmacology. 1982; 4:243–52.
Article4. Nemlander A, Ha ¨yry P. Effect of cyclosporin A on the generation of cytotoxic T lymphocytes in mouse mixed lymphocyte culture. Scand J Immunol. 1980; 12:493–8.
Article5. Alberti S, Boraschi D, Luini W, Tagliabue A. Effects of in vivo treatments with cyclosporin-A on mouse cell-mediated immune responses. Int J Immunopharmacol. 1981; 3:357–64.
Article6. Friedlaender GE. Immune responses to osteochondral allografts. Current knowledge and future directions. Clin Orthop Relat Res. 1983; 174:58–68.7. Friedlaender GE. Bone allografts: the biological consequences of immunological events. J Bone Joint Surg Am. 1991; 73:1119–22.8. Horowitz MC, Friedlaender GE. Immunologic aspects of bone transplantation. A rationale for future studies. Orthop Clin North Am. 1987; 18:227–33.
Article9. Horowitz MC, Friedlaender GE. Induction of specific T-cell responsiveness to allogeneic bone. J Bone Joint Surg Am. 1991; 73:1157–68.
Article10. Stevenson S. The immune response to osteochondral allografts in dogs. J Bone Joint Surg Am. 1987; 69:573–82.
Article11. Stevenson S, Horowitz M. The response to bone allografts. J Bone Joint Surg Am. 1992; 74:939–50.
Article12. Ekelund AL, Nilsson O. Effects of cyclosporin A on bone turnover and on resorption of demineralized bone matrix. Clin Orthop Relat Res. 1996; 326:127–34.
Article13. Frame JW. A convenient animal model for testing bone substitute materials. J Oral Surg. 1980; 38:176–80.14. Casser-Bette M, Murray AB, Closs EI, Erfle V, Schmidt J. Bone formation by osteoblast-like cells in a three-dimensional cell culture. Calcif Tissue Int. 1990; 46:46–56.
Article15. Bhargava U, Bar-Lev M, Bellows CG, Aubin JE. Ultrastructural analysis of bone nodules formed in vitro by isolated fetal rat calvaria cells. Bone. 1988; 9:155–63.
Article16. Nagata T, Bellows CG, Kasugai S, Butler WT, Sodek J. Biosynthesis of bone proteins [SPP-1 (secreted phosphoprotein-1, osteopontin), BSP (bone sialoprotein) and SPARC (osteonectin)] in association with mineralized-tissue formation by fetal-rat calvarial cells in culture. Biochem J. 1991; 274:513–20.
Article17. Ellis CN, Fradin MS, Messana JM, Brown MD, Siegel MT, Hartley AH, et al. Cyclosporine for plaque-type psoriasis. Results of a multidose, double-blind trial. N Engl J Med. 1991; 324:277–84.18. Green CJ. Experimental transplantation and cyclosporine. Transplantation. 1988; 46(2 Suppl):3S–10S.
Article19. Tugwell P, Bombardier C, Gent M, Bennett KJ, Bensen WG, Carette S, et al. Low-dose cyclosporin versus placebo in patients with rheumatoid arthritis. Lancet. 1990; 335:1051–5.
Article20. Boland J, Atkinson K, Britton K, Darveniza P, Johnson S, Biggs J. Tissue distribution and toxicity of cyclosporin A in the mouse. Pathology. 1984; 16:117–23.21. Shi CL, Rooth P, Ta ¨ljedal IB. Effects of cyclosporin A and verapamil on mouse pancreatic islets. Acta Endocrinol (Copenh). 1993; 129:54–8.
Article22. Fabien NH, Auger C, Moreira A, Monier JC. Effects of cyclosporin A on mouse thymus: immunochemical and ultrastructural studies. Thymus. 1992; 20:153–62.23. Aramant R, Turner JE. Cross-species grafting of embryonic mouse and grafting of older postnatal rat retinas into the lesioned adult rat eye: the importance of cyclosporin A for survival. Brain Res. 1988; 469:303–7.
Article24. Lems SP, Capel PJ, Koene RA. Rejection of long-surviving mouse skin allografts after withdrawal of cyclosporin A therapy. Transplant Proc. 1980; 12:283–6.25. Autenried P, Halloran PF. Cyclosporine blocks the induction of class I and class IIMHC products in mouse kidney by graft-vs-host disease. J Immunol. 1985; 135:3922–8.26. Halloran PF, Lee EH, Ziv I, Langer F, Gross AE. Orthotopic bone transplantation in mice. II. Studies of the alloantibody response. Transplantation. 1979; 27:420–6.27. Urist MR, Silverman BF, Bu ¨ring K, Dubuc FL, Rosenberg JM. The bone induction principle. Clin Orthop Relat Res. 1967; 53:243–83.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effect of Cyclosporin A on Osteoblast in vitro
- Comparison of Tacrolimus and Cyclosporine in Kidney Transplantation
- Fine structures of bone marrow cells in cyclosporine: A treated mouse
- The Fate of Calvarial Bone Graft in Nasal Tip Plasty Patients Followed Up for Over 10 Years
- Use of the Autogenous Calvarial Bone in Craniofacial Bone Graft