J Korean Acad Oral Health.
2014 Sep;38(3):148-153. 10.11149/jkaoh.2014.38.3.148.
Evaluation of the safety of non-thermal atmospheric-pressure plasma in hairless mouse tissues
- Affiliations
-
- 1Department of Dental Hygiene, Kyungnam College of Information & Technology, Busan, Korea. kjy1@kit.ac.kr
- 2Department of Oral Anatomy, School of Dentistry, Pusan National University, Yangsan, Korea.
- 3Department of Dental Hygiene, Dongseo University, Busan, Korea.
- KMID: 2321453
- DOI: http://doi.org/10.11149/jkaoh.2014.38.3.148
Abstract
OBJECTIVES
The aim of the present study was to evaluate the stability of non-thermal atmospheric-pressure plasma on Candida albicans in hairless mouse-2 (HRM-2) tissues.
METHODS
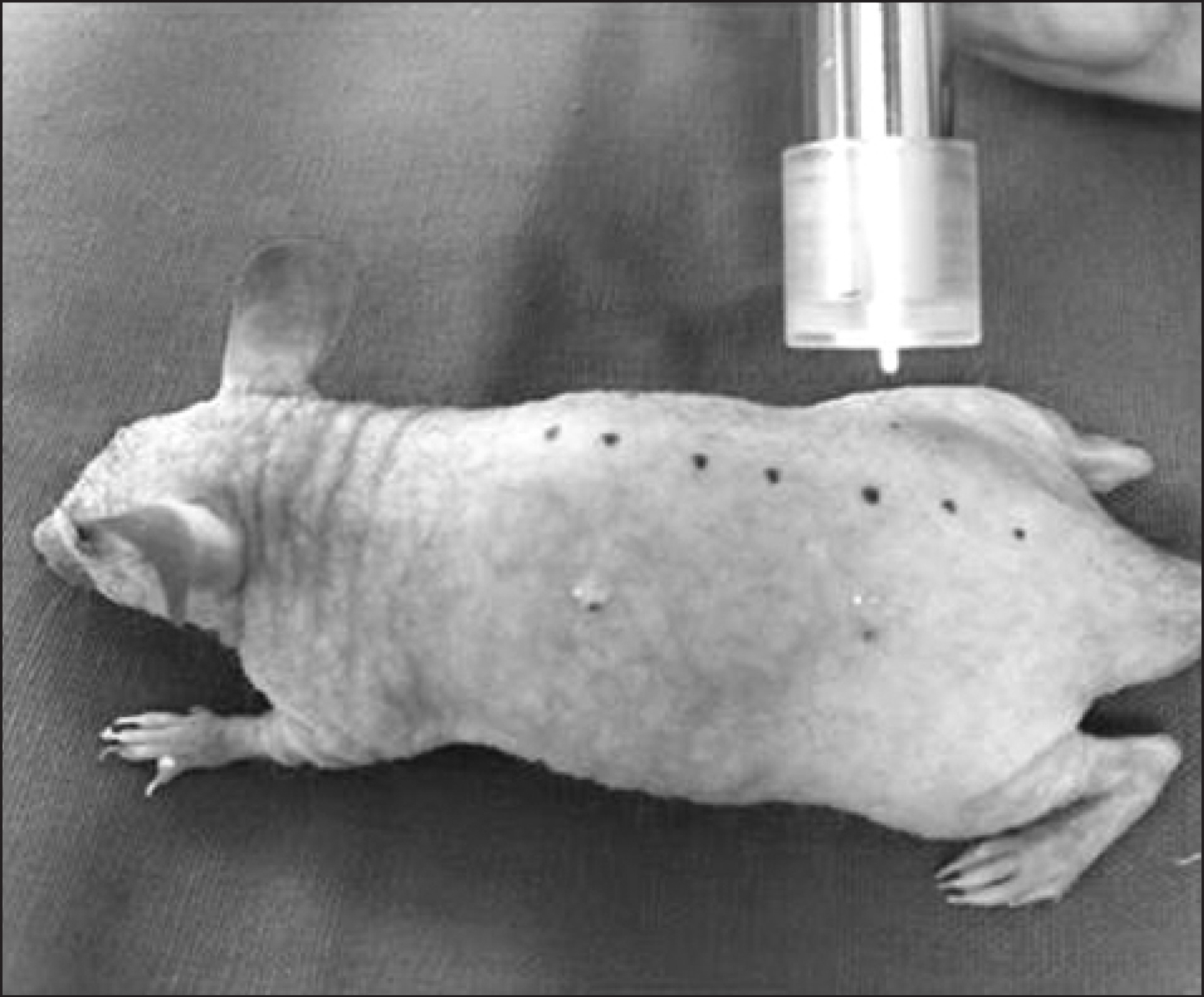
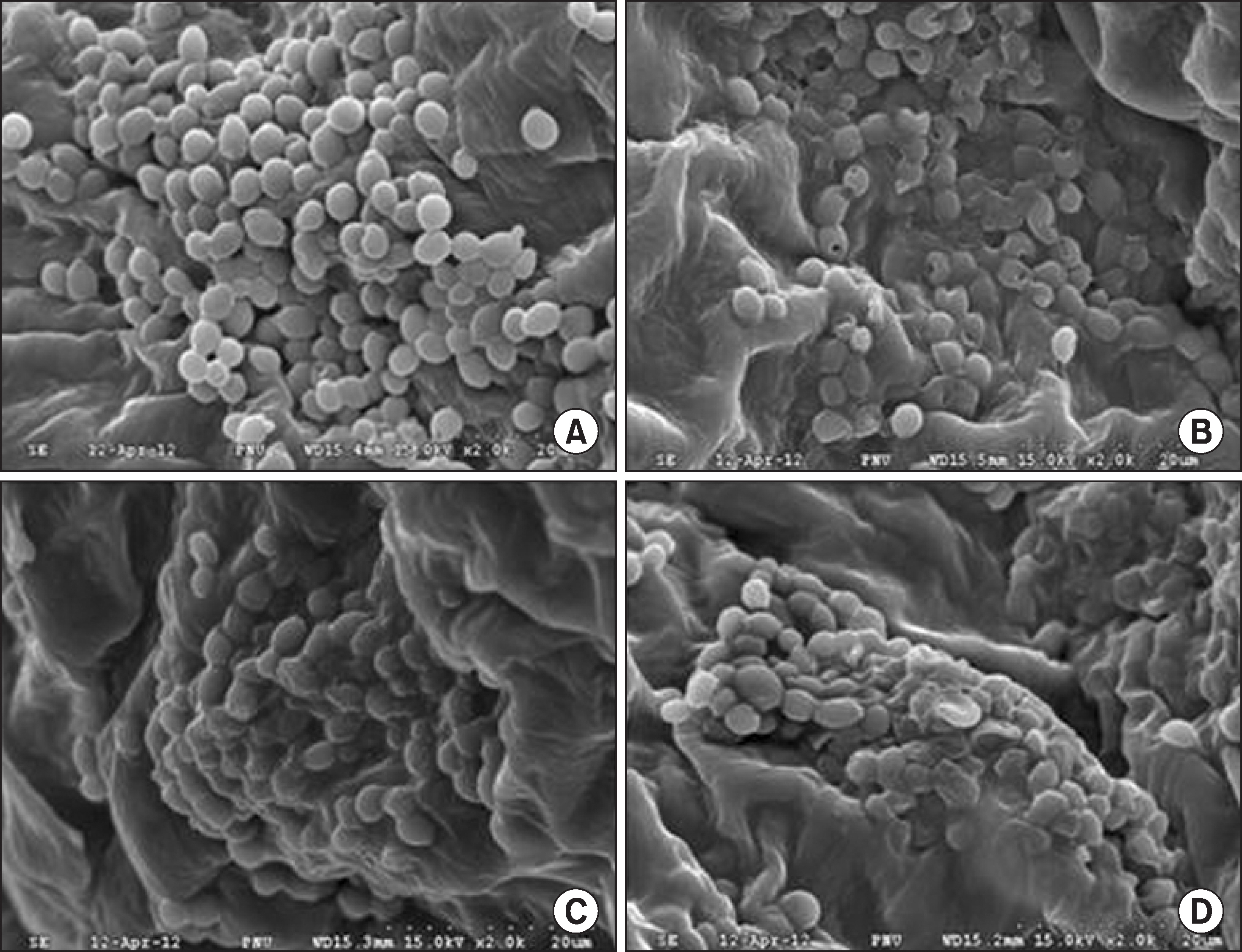
HRM-2 mice were subjected to non-thermal atmospheric-pressure plasma jet treatment using an optical fiber probe and monitored using a thermometer. The skin of HRM-2 mice was treated with plasma jet for 0, 60, 180, and 300 s per day for 5 days. After plasma treatment, morphological changes in Candida albicans on the skin of these mice were examined using a scanning electron microscope. Biopsy of the plasma-treated skin was performed and the tissues were histologically analyzed using hematoxylin and eosin (H&E) and Masson's trichrome stains.
RESULTS
The scanning electron microscopic images revealed the morphological changes in the membrane structure of the plasma-treated Candida albicans. Histological analysis showed that non-thermal plasma treatment did not cause epidermal damage or tissue inflammation and did not significantly modify the collagen layers of the mouse skin.
CONCLUSIONS
The results of this study suggest that non-thermal atmospheric-pressure plasma might be safe and effective for clinical applications in the field of dentistry.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Seo JD, Lee DK, Yim CJ. A study of effect of several gargling agents on normal oral microbial flora. J Korean Assoc Oral Maxil-lofac Surg. 1989; 15:96–102.2. Meitner SW, Bowen WH, Haidaris CG. Oral and esophageal Candida albicans infection in hyposalivatory rats. Infect Immun. 1990; 58:2228–2236.
Article3. Sitheeque MA, Samaranayake LP. Chronic hyperplastic candi-dosis/candidiasis (candidal leukoplakia). Crit Rev Oral Biol Med. 2003; 14:253–267.
Article4. Sanjaya PR, Gokul S, Gururaj Patil B, Raju R. Candida in oral pre-cancer and oral cancer. Med Hypotheses. 2011; 77:1125–1128.
Article5. Rupf S, Lehmann A, Hannig M, Schafer B, Schubert A, Feldmann U, et al. Killing of adherent oral microbes by a non-thermal atmospheric plasma jet. J Med Microbiol. 2010; 59:206–212.
Article6. Fridman G, Peddinghaus M, Ayan H, Fridman A, Balasubramanian Gutsol A, et al. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier. Plasma Chem Plasma Process. 2006; 26:425–442.7. Kim CH. New Conversing Technology; Plasma Medicine. Korean J Otorhinolaryngol-Head Neck Surg. 2010; 53:593–602.
Article8. Kim GJ, Kim W, Kim KT, Lee JK. DNA damage and mitochondria dysfunction in cell apoptosis induced by nonthermal air plasma. Appl Phys Lett. 2010; 96(021502):1–3.
Article9. Kim CH, Bahn JH, Lee SH, Kim GY, Sun JI, Lee K, et al. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J Biotechnol. 2010; 150:530–538.
Article10. Kang SK, Choi MY, Koo IG, Kim PY, Kim Y, Kim GJ, et al. Reactive hydroxyl radical-driven oral bacterial inactivation by radio frequency atmospheric plasma. Appl Phys Lett. 2011; 98(143702):1–3.
Article11. Lee HW, Kim GJ, Kim JM, Park JK, Lee JK, Kim GC. Tooth bleaching with nonthermal atmospheric pressure plasma. J En-dod. 2009; 35:587–591.
Article12. Nam SH, Lee HW, Cho SH, Lee JK, Jeon YC, Kim GC. High-efficiency tooth bleaching using non-thermal atmospheric pressure plasma with low concentration of hydrogen peroxide. J Appl Oral Sci. 2013; 21:265–270.
Article13. Kalghatgi SU, Fridman G, Cooper M, Nagaraj G, Peddinghaus M, Balasubramanian M, et al. Mechanism of Blood Coagulation by Nonthermal Atmospheric Pressure Dielectric Barrier Discharge Plasma. Plasma Science, IEEE Trans. 2007; 35:1559–1566.
Article14. Lloyd G, Friedman G, Jafri S, Schultz G, Fridman A, Harding K. Gas plasma: medical uses and developments in wound care. Plasma Processes Polym. 2010; 7:194–211.
Article15. Park SR, Kim GC. The killing effect of candida albicans on hairless mouse-2 mouse tissues by non-thermal atmospheric pressure plasma. J Dent Hyg Sci. 2014; 14:1–6.16. Choi J, Iza F, Do HJ, Lee JK, Cho MH. Microwave-excited atmospheric-pressure microplasmas based on a coaxial transmission line resonator. Plasma Sources Sci Technol. 2009; 18:1–8.
Article17. Park SJ, Choi J, Park GY, Lee SK, Cho YS, Yun JI, et al. Inactivation of S. mutans using an atmospheric plasma driven by a palm-size-integrated microwave power module. IEEE Trans Plasma Sci. 2010; 38:1956–1962.18. Dieterich C, Schandar C, Nolla MM, Johannes FJH, Graeve T, Rupp S. In vitro reconstructed human epithelia reveal contributions of Candida albicans EFG1 and CPH1 to adhesion and invasion. Microbiology. 2002; 148:497–506.
Article19. Cole GT, Halawa AA, Anaissie EJ. The role of the gastrointestinal tract in hematogenous candidiasis: from the laboratory to the bedside. Clin Infect Dis. 1996; 22:73–88.
Article20. Cutler JE. Putative virulence factors of Candida albicans. Annu Rev Microbiol. 1991; 45:187–218.
Article21. Matthews RC. Pathogenicity determinant of candida albicans: potential targets for immunotherapy? Microbiology. 1994; 140:1505–1511.22. Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A. Applied plasma medicine. Plasma Process Polym. 2008; 5:503–533.
Article23. Mendis DA, Rosenberg M, Azam F. A note on the possible electrostatic disruption of bacteria. IEEE Trans. Plasma Sci. 2000; 28:1304–1306.24. Laroussi M, Mendis DA, Rosenberg M. Plasma interaction with microbes. New J Phys. 2003; 5:41.1–41.10.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum: Evaluation of the safety of non-thermal atmospheric-pressure plasma in hairless mouse tissues
- Non-Thermal Atmospheric-Pressure Plasma Possible Application in Wound Healing
- New Conversing Technology; Plasma Medicine
- Sterilization effect of atmospheric pressure non-thermal air plasma on dental instruments
- Inactivation Efficacy of a Non-thermal Atmospheric Pressure Plasma Generator against Mycobacterium tuberculosis