Tuberc Respir Dis.
2011 May;70(5):390-396.
The Effect of Hyaluronan Treatment in Endotoxemic Rats
- Affiliations
-
- 1Department of Diagnostic Radiology, Keimyung University School of Medicine, Daegu, Korea.
- 2Department of Pathology, Keimyung University School of Medicine, Daegu, Korea.
- 3Department of Internal Medicine, Keimyung University School of Medicine, Daegu, Korea. wichoi@dsmc.or.kr
Abstract
- BACKGROUND
Hyaluronan (HA) is an unbranched glycosaminoglycan. It has been proposed that HA acts as a vehicle for cytokines due to the strong negative charge on its surface. We hypothesized that HA would function like a cytokine scavenger and reduce the inflammatory signaling cascade and this would lead to improved survival in rats suffering with endotoxemia.
METHODS
Endotoxin (Salmonella, 10 mg/kg) or an equal amount of 0.9% NaCl (NS) was injected into the jugular vein of rats. HA (1,600 kDa, 0.35%) or NS was given at 0.1 mL/kg/h for 3 hours. HA or NS infusion was started at 4 hour after endotoxin injection. The rats were divided into the control and HA groups (n=16 for each group). The mean arterial pressure (MAP) was monitored during HA or normal saline infusion. Survival was assessed every 12 hours for 3 days throughout the experiment.
RESULTS
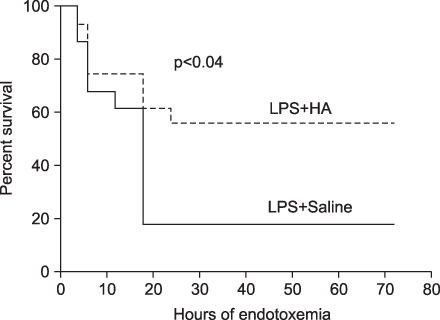
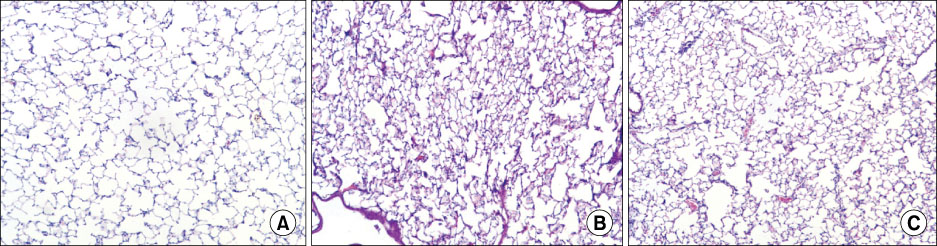
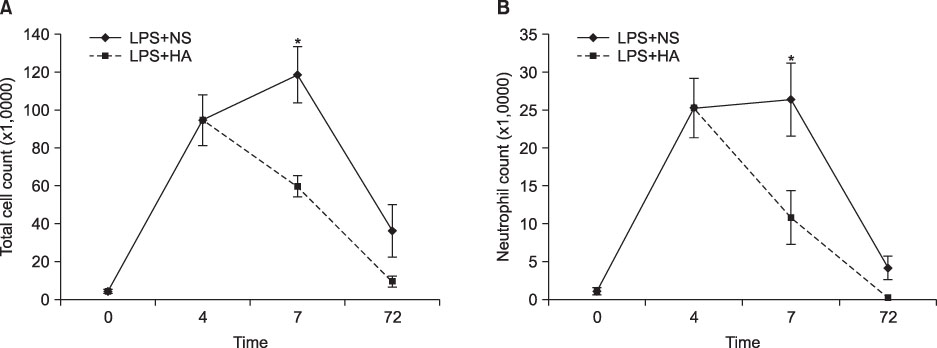
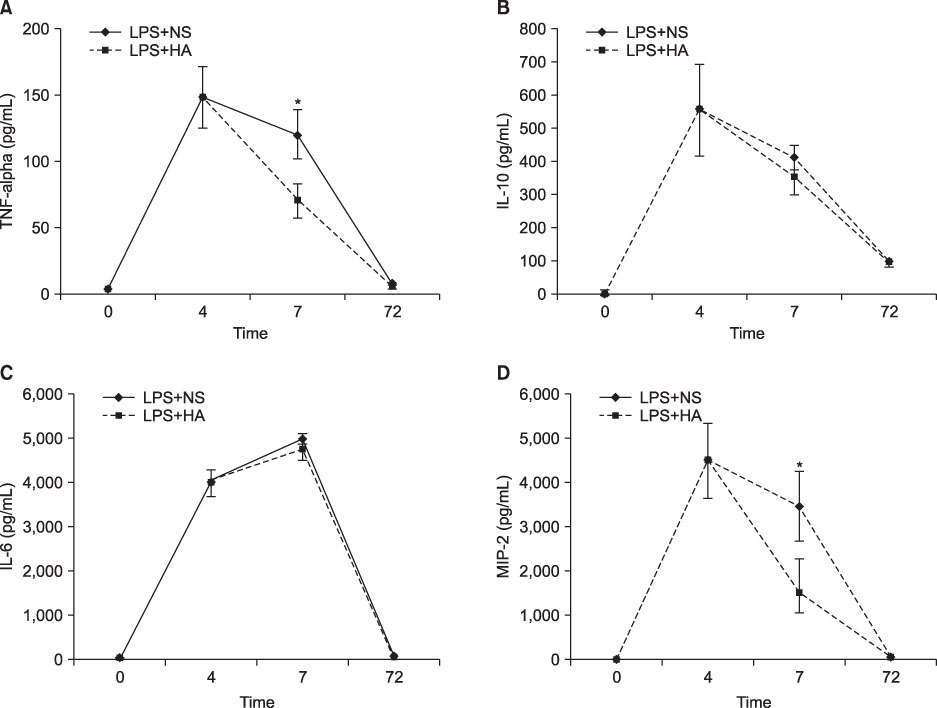
The survival rate (%) of the rats treated with HA was higher (60%) than that of the controls (20%) when HA was infused 4 hours after lipopolysaccharide (LPS) injection. The bronchoalveolar lavage (BAL) fluid of the animals surviving HA or NS infusion 4 hours after LPS showed that the total cell counts and number of neutrophils were significantly (p<0.01) reduced in the HA treated groups compared with that of the controls (total cell count, 9.2x10(4)/mL vs. 61x10(4)/mL; neutrophils, 21x10(4)/mL vs. 0.2x10(4)/mL, respectively). There was no significant MAP difference between the HA or control groups either with or without endotoxin.
CONCLUSION
Infusion of hyaluronan (1,600 kDa) reduced the BAL total cell count and the number of neutrophils and it improved the survival rate of the endotoxemic rats.
Keyword
MeSH Terms
Figure
Reference
-
1. Rangel-Frausto MS, Pittet D, Costigan M, Hwang T, Davis CS, Wenzel RP. The natural history of the systemic inflammatory response syndrome (SIRS). A prospective study. JAMA. 1995. 273:117–123.2. Irish Critical Care Trials Group. Acute lung injury and the acute respiratory distress syndrome in Ireland: a prospective audit of epidemiology and management. Crit Care. 2008. 12:R30.3. Starcher BC. Lung elastin and matrix. Chest. 2000. 117:5 Suppl 1. 229S–234S.4. Hance AJ, Crystal RG. The connective tissue of lung. Am Rev Respir Dis. 1975. 112:657–711.5. Cantor JO, Cerreta JM, Keller S, Turino GM. Modulation of airspace enlargement in elastase-induced emphysema by intratracheal instillment of hyaluronidase and hyaluronic acid. Exp Lung Res. 1995. 21:423–436.6. McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, et al. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996. 98:2403–2413.7. Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005. 11:1173–1179.8. Brandt KD, Smith GN Jr, Simon LS. Intraarticular injection of hyaluronan as treatment for knee osteoarthritis: what is the evidence? Arthritis Rheum. 2000. 43:1192–1203.9. Asari A, Miyauchi S, Matsuzaka S, Ito T, Kominami E, Uchiyama Y. Molecular weight-dependent effects of hyaluronate on the arthritic synovium. Arch Histol Cytol. 1998. 61:125–135.10. Nakamura K, Yokohama S, Yoneda M, Okamoto S, Tamaki Y, Ito T, et al. High, but not low, molecular weight hyaluronan prevents T-cell-mediated liver injury by reducing proinflammatory cytokines in mice. J Gastroenterol. 2004. 39:346–354.11. Cantor JO, Cerreta JM, Ochoa M, Ma S, Chow T, Grunig G, et al. Aerosolized hyaluronan limits airspace enlargement in a mouse model of cigarette smoke-induced pulmonary emphysema. Exp Lung Res. 2005. 31:417–430.12. Quinn DA, Moufarrej R, Volokhov A, Syrkina O, Hales CA. Combined smoke inhalation and scald burn in the rat. J Burn Care Rehabil. 2003. 24:208–216.13. Choi WI, Kwon KY, Kim JM, Quinn DA, Hales CA, Seo JW. Atelectasis induced by thoracotomy causes lung injury during mechanical ventilation in endotoxemic rats. J Korean Med Sci. 2008. 23:406–413.14. Bhatia RK, Pallister I, Dent C, Jones SA, Topley N. Enhanced neutrophil migratory activity following major blunt trauma. Injury. 2005. 36:956–962.15. Day AJ, de la Motte CA. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 2005. 26:637–643.16. Alam CA, Seed MP, Freemantle C, Brown J, Perretti M, Carrier M, et al. The inhibition of neutrophil-endothelial cell adhesion by hyaluronan independent of CD44. Inflammopharmacology. 2005. 12:535–550.17. Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007. 170:1435–1444.18. Cain BS, Meldrum DR, Dinarello CA, Meng X, Joo KS, Banerjee A, et al. Tumor necrosis factor-alpha and interleukin-1beta synergistically depress human myocardial function. Crit Care Med. 1999. 27:1309–1318.19. van der Poll T, Lowry SF. Tumor necrosis factor in sepsis: mediator of multiple organ failure or essential part of host defense? Shock. 1995. 3:1–12.20. Reinhart K, Menges T, Gardlund B, Harm Zwaveling J, Smithes M, Vincent JL, et al. Randomized, placebo-controlled trial of the anti-tumor necrosis factor antibody fragment afelimomab in hyperinflammatory response during severe sepsis: the RAMSES Study. Crit Care Med. 2001. 29:765–769.21. Macias WL, Nelson DR, Williams M, Garg R, Janes J, Sashegyi A. Lack of evidence for qualitative treatment by disease severity interactions in clinical studies of severe sepsis. Crit Care. 2005. 9:R607–R622.22. Scumpia PO, Moldawer LL. Biology of interleukin-10 and its regulatory roles in sepsis syndromes. Crit Care Med. 2005. 33:12 Suppl. S468–S471.23. Schmal H, Shanley TP, Jones ML, Friedl HP, Ward PA. Role for macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J Immunol. 1996. 156:1963–1972.24. Standiford TJ, Kunkel SL, Greenberger MJ, Laichalk LL, Strieter RM. Expression and regulation of chemokines in bacterial pneumonia. J Leukoc Biol. 1996. 59:24–28.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Carboxy Methyl Chitosan on Experimental Osteoarthritis in a Rabbit Knee: Carboxy methyl chitosan vs. Hyaluronan
- The effect of hyaluronan on osteogenesis in allogeneic bone grafting
- The Effect of Sevoflurane and Propofol on Expression of Inducible Nitric Oxide Synthase in Endotoxemic Rats
- Effects of Hyaluronan on Proliferation and Differentiation Cultured Chondrocyte
- Effect of Topical 1% Na-Hyaluronan on Basan Cell Morphogenesis in Corneal Wound Healing