Tuberc Respir Dis.
2010 Apr;68(4):218-225.
Differential Cell Analysis and Lymphocyte Subset Analysis in Bronchoalveolar Lavage Fluid from Patients with Miliary Tuberculosis
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Pusan National University School of Medicine, Yangsan, Korea. yskimdr@yahoo.co.kr
Abstract
- BACKGROUND
Bronchoalveolar lavage (BAL) is a useful technique to recover lower airway fluid and cells involved in many respiratory diseases. Miliary tuberculosis is potentially lethal, but the clinical manifestations are nonspecific and typical radiologic findings may not be seen until late in the course of disease. In addition, invasive procedures are often needed to confirm disease diagnosis. This study analyzed the cells and the T-lymphocyte subset in BAL fluid from patients with miliary tuberculosis to determine specific characteristics of BAL fluid that may help in the diagnosis of miliary tuberculosis, using a less invasive procedure.
METHODS
On a retrospective basis, we enrolled 20 miliary tuberculosis patients; 12 patients were male and the mean patient age was 40.5+/-16.2 years. We analyzed differential cell counts of BAL fluid and the T-lymphocyte subset of BAL fluid.
RESULTS
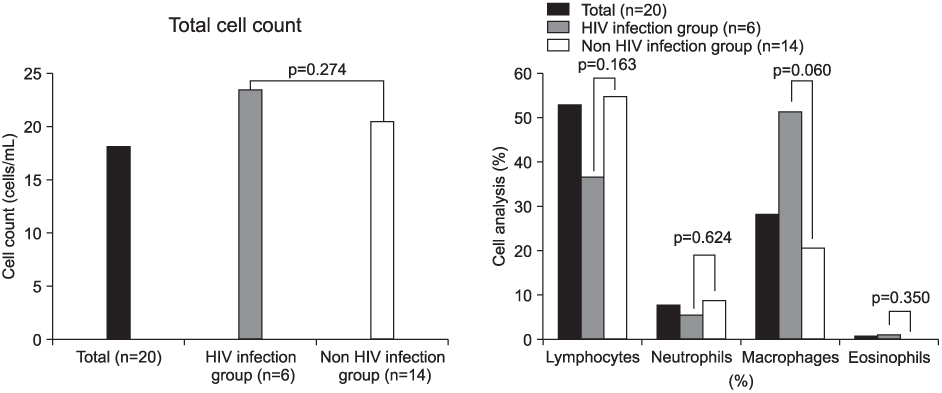
Total cells and lymphocytes were increased in number in the BAL fluid. The percentage of CD4+ T-lymphocytes and the CD4/CD8 ratio in BAL fluid were significantly decreased and the percentage of CD8+ T-lymphocytes was relatively higher. These findings were more prominent in patients infected with the human immunodeficiency virus (HIV). In the HIV-infected patients, the proportion of lymphocytes was significantly higher in BAL fluid than in peripheral blood. There were no significant differences between the BAL fluid and the peripheral blood T-lymphocytes subpopulation.
CONCLUSION
BAL fluid in patients with miliary tuberculosis demonstrated lymphocytosis, a lower percentage of CD4+ T-lymphocytes, a higher percentage of CD8+ T-lymphocytes, and a decreased CD4/CD8 ratio. These findings were more significant in HIV-infected subjects.
Keyword
MeSH Terms
Figure
Reference
-
1. Hunninghake GW, Gadek JE, Kawanami O, Ferrans VJ, Crystal RG. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979. 97:149–206.2. Crystal RG, Reynolds HY, Kalica AR. Bronchoalveolar lavage: the report of an international conference. Chest. 1986. 90:122–131.3. Daniele RP, Elias JA, Epstein PE, Rossman MD. Bronchoalveolar lavage: role in the pathogenesis, diagnosis, and management of interstitial lung disease. Ann Intern Med. 1985. 102:93–108.4. Bitterman PB, Rennard SI, Hunninghake GW, Crystal RG. Human alveolar macrophage growth factor for fibroblasts: regulation and partial characterization. J Clin Invest. 1982. 70:806–822.5. Hunninghake GW, Gadek JE, Lawley TJ, Crystal RG. Mechanisms of neutrophil accumulation in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest. 1981. 68:259–269.6. Reynolds HY, Fulmer JD, Kazmierowski JA, Roberts WC, Frank MM, Crystal RG. Analysis of cellular and protein content of broncho-alveolar lavage fluid from patients with idiopathic pulmonary fibrosis and chronic hypersensitivity pneumonitis. J Clin Invest. 1977. 59:165–175.7. Haslam PL, Turton CW, Heard B, Lukoszek A, Collins JV, Salsbury AJ, et al. Bronchoalveolar lavage in pulmonary fibrosis: comparison of cells obtained with lung biopsy and clinical features. Thorax. 1980. 35:9–18.8. Hunninghake GW, Kawanami O, Ferrans VJ, Young RC Jr, Roberts WC, Crystal RG. Characterization of the inflammatory and immune effector cells in the lung parenchyma of patients with interstitial lung disease. Am Rev Respir Dis. 1981. 123:407–412.9. Campbell DA, Poulter LW, du Bois RM. Immunocompetent cells in bronchoalveolar lavage reflect the cell populations in transbronchial biopsies in pulmonary sarcoidosis. Am Rev Respir Dis. 1985. 132:1300–1306.10. Semenzato G, Chilosi M, Ossi E, Trentin L, Pizzolo G, Cipriani A, et al. Bronchoalveolar lavage and lung histology: comparative analysis of inflammatory and immunocompetent cells in patients with sarcoidosis and hypersensitivity pneumonitis. Am Rev Respir Dis. 1985. 132:400–404.11. Turner-Warwick M, McAllister W, Lawrence R, Britten A, Haslam PL. Corticosteroid treatment in pulmonary sarcoidosis: do serial lavage lymphocyte counts, serum angiotensin converting enzyme measurements, and gallium-67scans help management? Thorax. 1986. 41:903–913.12. Turner-Warwick M, Haslam PL. The value of serial bronchoalveolar lavages in assessing the clinical progress of patients with cryptogenic fibrosing alveolitis. Am Rev Respir Dis. 1987. 135:26–34.13. Ozaki T, Nakahira S, Tani K, Ogushi F, Yasuoka S, Ogura T. Differential cell analysis in bronchoalveolar lavage fluid from pulmonary lesions of patients with tuberculosis. Chest. 1992. 102:54–59.14. Mert A, Bilir M, Tabak F, Ozaras R, Ozturk R, Senturk H, et al. Miliary tuberculosis: clinical manifestations, diagnosis and outcome in 38 adults. Respirology. 2001. 6:217–224.15. Mason RJ, Broaddus VC, Murray JF, Nadel JA. Murray and Nadel's textbook of respiratory medicine. 2005. 4th ed. Philadelphia: Elsevier Saunders.16. Turner-Warwick M, Haslam PL. Clinical applications of bronchoalveolar lavage. Clin Chest Med. 1987. 8:15–26.17. Ceuppens JL, Lacquet LM, Mariën G, Demedts M, van den Eeckhout A, Stevens E. Alveolar T-cell subsets in pulmonary sarcoidosis: correlation with disease activity and effect of steroid treatment. Am Rev Respir Dis. 1984. 129:563–568.18. Godard P, Clot J, Jonquet O, Bousquet J, Michel FB. Lymphocyte subpopulations in bronchoalveolar lavages of patients with sarcoidosis and hypersensitivity pneumonitis. Chest. 1981. 80:447–452.19. Leatherman JW, Michael AF, Schwartz BA, Hoidal JR. Lung T cells in hypersensitivity pneumonitis. Ann Intern Med. 1984. 100:390–392.20. Hunninghake GW, Crystal RG. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med. 1981. 305:429–434.21. Collins FM. The immunology of tuberculosis. Am Rev Respir Dis. 1982. 125:42–49.22. Daniel TM. The immunology of tuberculosis. Clin Chest Med. 1980. 1:189–201.23. Chaparas SD. The immunology of mycobacterial infections. Crit Rev Microbiol. 1982. 9:139–197.24. Kim DS, Lee BC. Changes in cell patterns of bronchoalveolar lavage fluid in active pulmonary tuberculosis. Korean J Intern Med. 1988. 34:12–19.25. Han SK, Cho SH, Kim JW, Kim YW, Shim YS, Kim KY, et al. The changes of T-lymphocyte and its subsets in the bronchoalveolar lavage fluid (BALF) and peripheral blood (PB) of pulmonary tuberculosis patients. Korean J Intern Med. 1988. 34:285–293.26. Ainslie GM, Solomon JA, Bateman ED. Lymphocyte and lymphocyte subset numbers in blood and in bronchoalveolar lavage and pleural fluid in various forms of human pulmonary tuberculosis at presentation and during recovery. Thorax. 1992. 47:513–518.27. Rook GA. The immunological consequences of antigen overload in experimental mycobacterial infections of mice. Clin Exp Immunol. 1975. 19:167–177.28. Rook GA, Champion BR, Steele J, Varey AM, Stanford JL. I-A restricted activation by T cell lines of anti-tuberculosis activity in murine macrophages. Clin Exp Immunol. 1985. 59:414–420.29. Keusch GT, Wilson CS, Waksal SD. Gallin JI, Fauci AS, editors. Nutrition, host defenses, and the lymphoid system. Advances in host defense mechanisms. 1983. New York: Raven Press;275–359.30. Chandra RK. Lymphocyte subpopulations in human malnutrition: cytotoxic and suppressor cells. Pediatrics. 1977. 59:423–427.31. Tsao TC, Chen CH, Hong JH, Hsieh MJ, Tsao KC, Lee CH. Shifts of T4/T8 T lymphocytes from BAL fluid and peripheral blood by clinical grade in patients with pulmonary tuberculosis. Chest. 2002. 122:1285–1291.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparative Analysis of Bronchoalveolar Lavages in Interstitial Lung Diseases
- Changes of the cellularities in the bronchoalveolar lavage fluid of the experimental silicosis
- The Diagnostic Value of Bronchoalveolar lavage fluid microscopic study and PCR in Pulmonary tuberculosis
- Analysis of Bronchoalveolar Lavage Fluid cells from the Patients of Diffuse Interstitial Lung Diseases
- CD45 is Essential for Lymphocyte Gating in a T-lymphocyte Subset Assay of Bronchoalveolar Lavage Fluid by Flow Cytometry