Pediatr Gastroenterol Hepatol Nutr.
2013 Dec;16(4):207-218.
Long Term Outcomes after Pediatric Liver Transplantation
- Affiliations
-
- 1MedStar Georgetown Transplant Institute, Georgetown University Hospital, Washington DC, USA. nada.a.yazigi@gunet.georgetown.edu
Abstract
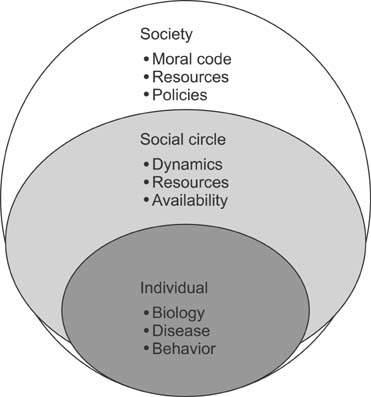
- Long term outcomes after liver transplantation are major determinants of quality of life and of the value of this heroic treatment. As short term outcomes are excellent, our community is turning to take a harder look at long term outcomes. The purpose of this paper is to review these outcomes, and highlight proposed treatments, as well as pressing topics needing to be studied. A systemic review of the English literature was carried in PubMed, covering all papers addressing long term outcomes in pediatric liver transplant from 2000-2013. Late outcomes after pediatric liver transplant affect the liver graft in the form of chronic liver dysfunction. The causes include rejection particularly humoral rejection, but also de novo autoimmune hepatitis, and recurrent disease. The metabolic syndrome is a major factor in long term cardiovascular complication risk. Secondary infections, kidney dysfunction and malignancy remain a reality of those patients. There is growing evidence of late cognitive and executive function delays affecting daily life productivity as well as likely adherence. Finally, despite a good health status, quality of life measures are comparable to those of children with chronic diseases. Long term outcomes are the new frontier in pediatric liver transplantation. Much is needed to improve graft survival, but also to avoid systemic morbidities from long term immunosuppression. Quality of life is a new inclusive measure that will require interventions and innovative approaches respectful not only on the patients but also of their social circle.
Keyword
MeSH Terms
Figure
Reference
-
1. Ng VL, Alonso EM, Bucuvalas JC, Cohen G, Limbers CA, Varni JW, et al. Studies of Pediatric Liver Transplantation (SPLIT) Research Group. Health status of children alive 10 years after pediatric liver transplantation performed in the US and Canada: report of the studies of pediatric liver transplantation experience. J Pediatr. 2012; 160:820–826.
Article2. Kelly DA, Bucuvalas JC, Alonso EM, Karpen SJ, Allen U, Green M, et al. Long-term medical management of the pediatric patient after liver transplantation: 2013 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver Transpl. 2013; 19:798–825.
Article3. Bekker J, Ploem S, de Jong KP. Early hepatic artery thrombosis after liver transplantation: a systematic review of the incidence, outcome and risk factors. Am J Transplant. 2009; 9:746–757.
Article4. Jensen MK, Campbell KM, Alonso MH, Nathan JD, Ryckman FC, Tiao GM. Management and long-term consequences of portal vein thrombosis after liver transplantation in children. Liver Transpl. 2013; 19:315–321.
Article5. Mazariegos GV. Withdrawal of immunosuppression in liver transplantation: lessons learned from PTLD. Pediatr Transplant. 2004; 8:210–213.
Article6. Kaneku H, O'Leary JG, Banuelos N, Jennings LW, Susskind BM, Klintmalm GB, et al. De novo donor-specific HLA antibodies decrease patient and graft survival in liver transplant recipients. Am J Transplant. 2013; 13:1541–1548.
Article7. Evans HM, Kelly DA, McKiernan PJ, Hübscher S. Progressive histological damage in liver allografts following pediatric liver transplantation. Hepatology. 2006; 43:1109–1117.
Article8. Ekong UD, Melin-Aldana H, Seshadri R, Lokar J, Harris D, Whitington PF, et al. Graft histology characteristics in long-term survivors of pediatric liver transplantation. Liver Transpl. 2008; 14:1582–1587.
Article9. Scheenstra R, Peeters PM, Verkade HJ, Gouw AS. Graft fibrosis after pediatric liver transplantation: ten years of follow-up. Hepatology. 2009; 49:880–886.
Article10. Liberal R, Zen Y, Mieli-Vergani G, Vergani D. Liver transplantation and autoimmune liver diseases. Liver Transpl. 2013; 19:1065–1077.
Article11. Desai M, Neuberger J. Chronic liver allograft dysfunction. Transplant Proc. 2009; 41:773–776.
Article12. Martin SR, Alvarez F, Anand R, Song C, Yin W. SPLIT Research Group. Outcomes in children who underwent transplantation for autoimmune hepatitis. Liver Transpl. 2011; 17:393–401.
Article13. Aguilera I, Sousa JM, Gavilan F, Gomez L, Alvarez-Márquez A, Núñez-Roldán A. Complement component 4d immunostaining in liver allografts of patients with de novo immune hepatitis. Liver Transpl. 2011; 17:779–788.
Article14. Gibelli NE, Tannuri U, Pinho-Apezzato ML, Tannuri AC, Maksoud-Filho JG, Andrade WC, et al. Sirolimus in pediatric liver transplantation: a single-center experience. Transplant Proc. 2009; 41:901–903.
Article15. Sutedja DS, Gow PJ, Hubscher SG, Elias E. Revealing the cause of cryptogenic cirrhosis by posttransplant liver biopsy. Transplant Proc. 2004; 36:2334–2337.
Article16. Campbell K, Ng V, Martin S, Magee J, Goebel J, Anand R, et al. SPLIT Renal Function Working Group. Glomerular filtration rate following pediatric liver transplantation--the SPLIT experience. Am J Transplant. 2010; 10:2673–2682.
Article17. Nobili V, de Ville de Goyet J. Pediatric post-transplant metabolic syndrome: new clouds on the horizon. Pediatr Transplant. 2013; 17:216–223.
Article18. Ng VL, Fecteau A, Shepherd R, Magee J, Bucuvalas J, Alonso E, et al. Studies of Pediatric Liver Transplantation Research Group. Outcomes of 5-year survivors of pediatric liver transplantation: report on 461 children from a north american multicenter registry. Pediatrics. 2008; 122:e1128–e1135.
Article19. Madhwal S, Atreja A, Albeldawi M, Lopez R, Post A, Costa MA. Is liver transplantation a risk factor for cardiovascular disease? A meta-analysis of observational studies. Liver Transpl. 2012; 18:1140–1146.
Article20. Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for persons aged 0 through 18 years-United States, 2013. MMWR Surveill Summ. 2013; 62:Suppl 1. 1.21. Codoner-Franch P, Bernard O, Alvarez F. Long-term follow-up of growth in height after successful liver transplantation. J Pediatr. 1994; 124:368–373.
Article22. Sebagh M, Samuel D, Antonini TM, Coilly A, Degli Esposti D, Roche B, et al. Twenty-year protocol liver biopsies: Invasive but useful for the management of liver recipients. J Hepatol. 2012; 56:840–847.
Article23. Heffron TG, Pillen T, Smallwood G, Rodriguez J, Sekar S, Henry S, et al. Pediatric liver transplantation for acute liver failure at a single center: a 10-yr experience. Pediatr Transplant. 2010; 14:228–232.
Article24. Lao OB, Dick AA, Healey PJ, Perkins JD, Reyes JD. Identifying the futile pediatric liver re-transplant in the PELD era. Pediatr Transplant. 2010; 14:1019–1029.
Article25. Feng S, Ekong UD, Lobritto SJ, Demetris AJ, Roberts JP, Rosenthal P, et al. Complete immunosuppression withdrawal and subsequent allograft function among pediatric recipients of parental living donor liver transplants. JAMA. 2012; 307:283–293.
Article26. Bohne F, Martínez-Llordella M, Lozano JJ, Miquel R, Benítez C, Londoño MC, et al. Intra-graft expression of genes involved in iron homeostasis predicts the development of operational tolerance in human liver transplantation. J Clin Invest. 2012; 122:368–382.
Article27. Tryphonopoulos P, Ruiz P, Weppler D, Nishida S, Levi DM, Moon J, et al. Long-term follow-up of 23 operational tolerant liver transplant recipients. Transplantation. 2010; 90:1556–1561.
Article28. Abdelmalek MF, Humar A, Stickel F, Andreone P, Pascher A, Barroso E, et al. Sirolimus Liver Conversion Trial Study Group. Sirolimus conversion regimen versus continued calcineurin inhibitors in liver allograft recipients: a randomized trial. Am J Transplant. 2012; 12:694–705.
Article29. Berquist RK, Berquist WE, Esquivel CO, Cox KL, Wayman KI, Litt IF. Adolescent non-adherence: prevalence and consequences in liver transplant recipients. Pediatr Transplant. 2006; 10:304–310.
Article30. Shemesh E, Lurie S, Stuber ML, Emre S, Patel Y, Vohra P, et al. A pilot study of posttraumatic stress and nonadherence in pediatric liver transplant recipients. Pediatrics. 2000; 105:E29.
Article31. Shemesh E, Annunziato RA, Arnon R, Miloh T, Kerkar N. Adherence to medical recommendations and transition to adult services in pediatric transplant recipients. Curr Opin Organ Transplant. 2010; 15:288–292.
Article32. Pollock-Barziv SM, Finkelstein Y, Manlhiot C, Dipchand AI, Hebert D, Ng VL, et al. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant. 2010; 14:968–975.
Article33. Fredericks EM, Dore-Stites D, Well A, Magee JC, Freed GL, Shieck V, et al. Assessment of transition readiness skills and adherence in pediatric liver transplant recipients. Pediatr Transplant. 2010; 14:944–953.
Article34. Huda A, Newcomer R, Harrington C, Blegen MG, Keeffe EB. High rate of unemployment after liver transplantation: analysis of the United Network for Organ Sharing database. Liver Transpl. 2012; 18:89–99.
Article35. Burra P, Germani G, Gnoato F, Lazzaro S, Russo FP, Cillo U, et al. Adherence in liver transplant recipients. Liver Transpl. 2011; 17:760–770.
Article36. Limbers CA, Neighbors K, Martz K, Bucuvalas JC, Webb T, Varni JW, et al. Studies of Pediatric Liver Transplantation Functional Outcomes Group. Health-related quality of life in pediatric liver transplant recipients compared with other chronic disease groups. Pediatr Transplant. 2011; 15:245–253.
Article37. Mohammad S, Hormaza L, Neighbors K, Boone P, Tierney M, Azzam RK, et al. Health status in young adults two decades after pediatric liver transplantation. Am J Transplant. 2012; 12:1486–1495.
Article38. Sorensen LG, Neighbors K, Martz K, Zelko F, Bucuvalas JC, Alonso EM. Studies of Pediatric Liver Transplantation (SPLIT) and Functional Outcomes Group (FOG). Cognitive and academic outcomes after pediatric liver transplantation: Functional Outcomes Group (FOG) results. Am J Transplant. 2011; 11:303–311.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Pediatric liver transplantation in Korea: long-term outcomes and allocations
- Issues on Long-term Management after Liver Transplantation in Children
- Pediatric Liver Transplantation
- Pediatric heart transplantation: how to manage problems affecting long-term outcomes?
- Indication of Pediatric Liver Transplantation