Korean J Urol.
2011 Sep;52(9):626-631.
Fungal Urinary Tract Infection in Burn Patients with Long-Term Foley Catheterization
- Affiliations
-
- 1Department of Urology, Hangang Sacred Heart Hospital, Hallym University College of Medicine, Seoul, Korea. ngchoi01@hallym.or.kr
Abstract
- PURPOSE
It is well known that fungi become predominant microorganisms in the urine of patients with long-term Foley catheters. This study was conducted to evaluate the lengths of time for fungi to cause urinary tract infection (UTI) and to identify predictors of fungal UTI in burn patients with long-term Foley catheters.
MATERIALS AND METHODS
A total of 93 patients who did not have infection at the time of admission but later had fugal UTI were evaluated. Urinalysis, urine culture, and Foley catheter indwelling were done at admission. All patients were administered prophylactic antibiotics from admission. Urine cultures were run every week, and catheters were changed every 2 weeks for each patient.
RESULTS
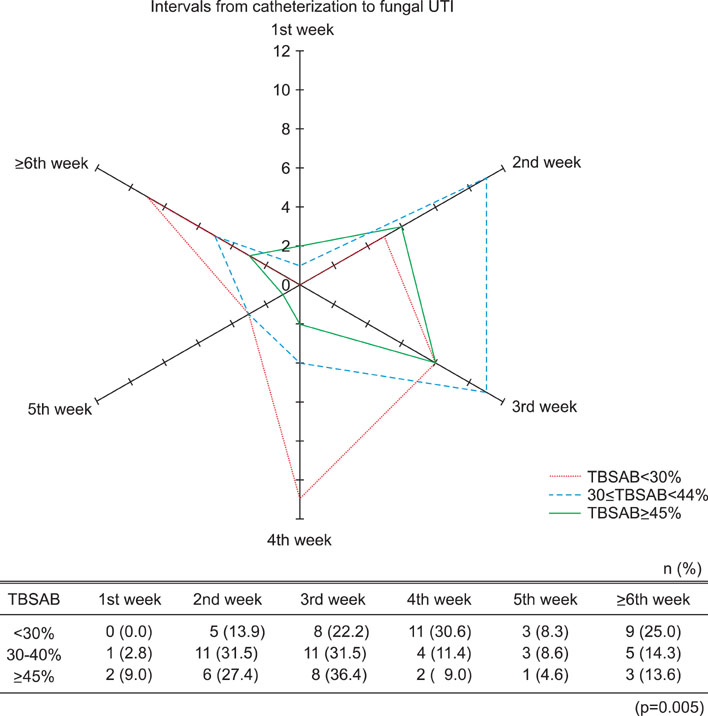
Three of the 93 patients (3.2%) displayed fungal UTI at the 1st week of catheter indwelling. However, most patients (78.5%) displayed fungal UTI from 2nd to 5th week after catheter indwelling. The most prevalent fungus identified was Candida tropicalis (60.2%). By univariate logistic regression analysis, only the total body surface area burned (TBSAB) was predictive of fungal UTI in burn patients (p=0.010). By multivariate logistic regression analysis, underlying disease (p=0.032) and TBSAB (p=0.036) were predictors of fungal UTI. Patients with higher TBSAB were more likely to display shorter intervals from Foley catheterization to fungal UTI.
CONCLUSIONS
Fungal UTI was initially found at the 1st week of urinary catheter indwelling, but the majority of cases occurred after the 1st week and appeared earlier in patients with underlying disease or higher TBSAB. Underlying disease and TBSAB were predictors of early fungal UTI.
MeSH Terms
Figure
Reference
-
1. Kunin CM, McCormack RC. Prevention of catheter-induced urinary tract infections by sterile closed drainage. N Engl J Med. 1966. 274:1155–1161.2. Maskell R, Pead L, Allen J. The puzzle of "urethral syndrome": a possible answer? Lancet. 1979. 1:1058–1059.3. Chan RC, Bruce AW, Reid G. Adherence of cervical, vaginal and distal urethral normal microbial flora to human uroepithelial cells and the inhibition of adherence of gram-negative uropathogens by competitive exclusion. J Urol. 1984. 131:596–601.4. Lundstrom T, Sobel J. Nosocomial candiduria: a review. Clin Infect Dis. 2001. 32:1602–1607.5. Sobel JD. Management of asymptomatic candiduria. Int J Antimicrob Agents. 1999. 11:285–288.6. Neaman KC, Andres LA, McClure AM, Burton ME, Kemmeter PR, Ford RD. A new method for estimation of involved BSAs for obese and normal-weight patients with burn injury. J Burn Care Res. 2011. 32:421–428.7. Pruitt BA Jr, McManus AT. Opportunistic infections in severely burned patients. Am J Med. 1984. 76:146–154.8. Manson WL, Pernot PC, Fidler V, Sauer EW, Klasen HJ. Colonization of burns and the duration of hospital stay of severely burned patients. J Hosp Infect. 1992. 22:55–63.9. Pruitt BA Jr, Lindberg RB, McManus WF, Mason AD Jr. Current approach to prevention and treatment of Pseudomonas aeruginosa infections in burned patients. Rev Infect Dis. 1983. 5:Suppl 5. S889–S897.10. Beck-Sagué CM, Jarvis WR. Secular trends in the epidemiology of nosocomial fungal infections in the United States, 1980-1990. The National Nosocomial Infections Surveillance System. J Infect Dis. 1993. 167:1247–1251.11. Burdge JJ, Rea F, Ayers L. Noncandidal, fungal infections of the burn wound. J Burn Care Rehabil. 1988. 9:599–601.12. Schofield CM, Murray CK, Horvath EE, Cancio LC, Kim SH, Wolf SE, et al. Correlation of culture with histopathology in fungal burn wound colonization and infection. Burns. 2007. 33:341–346.13. Luo G, Peng Y, Yuan Z, Cheng W, Wu J, Fitzgerald M. Yeast from burn patients at a major burn centre of China. Burns. 2011. 37:299–303.14. Atiyeh BS, Gunns W, Hayek SN. State of the art in burn treatment. World J Surg. 2005. 29:131–148.15. Church D, Elsayed S, Reid O, Winston B, Lindsay R. Burn wound infections. Clin Microbiol Rev. 2006. 19:403–434.16. Singh V, Devgan L, Bhat S, Milner SM. The pathogenesis of burn wound conversion. Ann Plast Surg. 2007. 59:109–115.17. Bruck HM, Nash G, Stein JM, Lindberg RB. Studies on the occurrence and significance of yeasts and fungi in the burn wound. Ann Surg. 1972. 176:108–110.18. Cawley MJ, Braxton GR, Haith LR, Reilly KJ, Guilday RE, Patton ML. Trichosporon beigelii infection: experience in a regional burn center. Burns. 2000. 26:483–486.19. Holzheimer RG, Dralle H. Management of mycoses in surgical patients - review of the literature. Eur J Med Res. 2002. 7:200–226.20. Richardson MD, Warnock DW. Richardson MD, Warnock DW, editors. Invasive candidosis. Fungal infection: diagnosis and management. 2003. 3rd ed. Oxford: Blackwell Scientific Publications;192–193.21. Kim DK, Yoo ES, Kim GN, Chung SK. Emphysematous cystitis with fungus Ball. Korean J Urol. 2002. 43:904–906.22. Lee SW. An aspergilloma mistaken for a pelviureteral stone on nonenhanced CT: a fungal bezoar causing ureteral obstruction. Korean J Urol. 2010. 51:216–218.23. Wise GJ, Silver DA. Fungal infections of the genitourinary system. J Urol. 1993. 149:1377–1388.24. Frye KR, Donovan JM, Drach GW. Torulopsis glabrata urinary infections: a review. J Urol. 1988. 139:1245–1249.25. Bell DA, Rose SC, Starr NK, Jaffe RB, Miller FJ Jr. Percutaneous nephrostomy for nonoperative management of fungal urinary tract infections. J Vasc Interv Radiol. 1993. 4:311–315.26. Sonda LP, Amendola MA. Candida pyocalix: unusual complication of prolonged nephrostomy drainage. J Urol. 1985. 134:722–724.27. Vordermark JS 2nd, Modarelli RO, Buck AS. Torulopsis pyelonephritis associated with papillary necrosis: a case report. J Urol. 1980. 123:96–97.28. Wainstein MA, Graham RC Jr, Resnick MI. Predisposing factors of systemic fungal infections of the genitourinary tract. J Urol. 1995. 154:160–163.29. Çakir B, Yeğan BÇ. Systemic responses to burn injury. Turk J Med Sci. 2004. 34:215–226.30. Tredget EE, Shankowsky HA, Rennie R, Burrell RE, Logsetty S. Pseudomonas infections in the thermally injured patient. Burns. 2004. 30:3–26.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Risk Factors for Nosocomial Urinary Tract Infection in the Intensive Care Unit with a Positive Urine Culture and Foley Catheterization
- Association between Asymptomatic Urinary Tract Infection and Postoperative Spine Infection in Elderly Women : A Retrospective Analysis Study
- Bladder Management after Total Hip Arthroplasty under Spinal Anesthesia
- Effects of perineal care in preventing catheter associated urinary tract infections (CAUTI) in intensive care units (ICU)
- Management of urinary tract infection in geriatric hospital patients