Korean J Urol.
2011 Nov;52(11):746-751.
Comparison of Ketoconazole and Estramustine for Treating Patients with Castration-Resistant Prostate Cancer
- Affiliations
-
- 1Department of Urology, Chonnam National University Medical School, Gwangju, Korea. urohwang@gmail.com
Abstract
- PURPOSE
We investigated the efficacy of ketoconazole and estramustine before chemotherapy for treating patients with progressive castration-resistant prostate cancer (CRPC) after anti-androgen withdrawal syndrome.
MATERIALS AND METHODS
Eighty-four patients who were diagnosed with CRPC and were treated between 2005 and 2009 were included. Thirty-nine patients were treated with 600 mg of ketoconazole and 10 mg of prednisolone per day (group I), and 45 patients were treated with 560 mg of estramustine per day (group II). The prostate-specific antigen (PSA) response, progression-free survival, and side effects were compared.
RESULTS
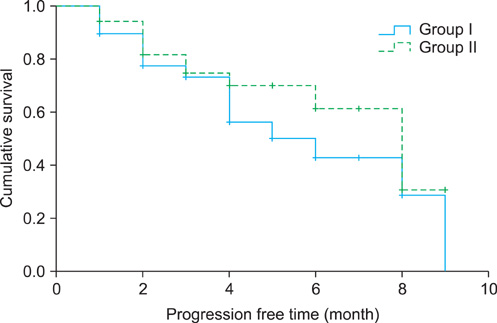
The median age of the patients, PSA level, and follow-up period were 72 years, 48.5 ng/ml, and 4 months (range, 1 to 29 months), respectively. The overall PSA response rate was 35.7%, and the PSA response rates were 33.3% for group I and 37.8% for group II (p=0.672). The median progression-free survival times were 8 months (95% confidence interval [CI] 5.9-10.1) overall, 5 months (95% CI 1.6-8.3) in group I, and 8 months (95% CI 5.9-10.0) in group II (p=0.282). The most common complications in groups I and II were nausea and vomiting (51.3%) and anemia (77.8%), respectively. Nausea and vomiting and hepatotoxicity were observed more often in group I, and gynecomastia, neutropenia, and anemia were observed more often in group II. The toxicities of each adverse effect were < or =grade 2.
CONCLUSIONS
With a resultant PSA decline and mild adverse effects, both ketoconazole and estramustine are worth consideration as treatment options for progressive CRPC patients after primary hormonal therapy.
Keyword
MeSH Terms
Figure
Reference
-
1. Ryan CJ, Small EJ. Role of secondary hormonal therapy in the management of recurrent prostate cancer. Urology. 2003. 62:Suppl 1. 87–94.2. Lam JS, Leppert JT, Vemulapalli SN, Shvarts O, Belldegrun AS. Secondary hormonal therapy for advanced prostate cancer. J Urol. 2006. 175:27–34.3. Scher HI, Halabi S, Tannock I, Morris M, Sternberg CN, Carducci MA, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008. 26:1148–1159.4. Choi BK, Park CH, Kim CI. Comparison of ketoconazole-prednisolone combination therapy with prednisolone alone in patients with hormone refractory prostate cancer. Korean J Urol. 2000. 41:1183–1189.5. Wilkinson S, Chodak G. An evaluation of intermediate-dose ketoconazole in hormone refractory prostate cancer. Eur Urol. 2004. 45:581–585.6. Small EJ, Halabi S, Dawson NA, Stadler WM, Rini BI, Picus J, et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J Clin Oncol. 2004. 22:1025–1033.7. Scholz M, Jennrich R, Strum S, Brosman S, Johnson H, Lam R. Long-term outcome for men with androgen independent prostate cancer treated with ketoconazole and hydrocortisone. J Urol. 2005. 173:1947–1952.8. Han KS, Cho KS, Lee SH, Hong SJ. Estramustine phosphate based chemotherapy for hormone refractory prostate cancer. Korean J Urol. 2007. 48:684–690.9. Hirano D, Minei S, Kishimoto Y, Yamaguchi K, Hachiya T, Yoshida T, et al. Prospective study of estramustine phosphate for hormone refractory prostate cancer patients following androgen deprivation therapy. Urol Int. 2005. 75:43–49.10. Trachtenberg J, Halpern N, Pont A. Ketoconazole: a novel and rapid treatment for advanced prostatic cancer. J Urol. 1983. 130:152–153.11. Stearns ME, Wang M, Tew KD, Binder LI. Estramustine binds a MAP-1-like protein to inhibit microtubule assembly in vitro and disrupt microtubule organization in DU 145 cells. J Cell Biol. 1988. 107:2647–2656.12. Iversen P, Rasmussen F, Asmussen C, Christensen IJ, Eickhoff J, Klarskov P, et al. Estramustine phosphate versus placebo as second line treatment after orchiectomy in patients with metastatic prostate cancer: DAPROCA study 9002. Danish Prostatic Cancer Group. J Urol. 1997. 157:929–934.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Dramatic Decline of PSA and Symptom Improvement after Estramustine Withdrawal in a Hormone-refractory Prostate Cancer Patient
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- Ketoconazole with Prednisolone for the Treatment of Hormone Refractory Prostate Cancer
- Precision Medicine in Castration-Resistant Prostate Cancer
- Estramustine Phosphate Monotherapy in Castration-Resistant Prostate Cancer Patients Who Cannot Receive Cytotoxic Chemotherapy