Pediatr Gastroenterol Hepatol Nutr.
2012 Jun;15(2):85-90.
Comparison of Four Commercial ELISA Kits and In-House Immunoblotting for Diagnosis of Helicobacter pylori Infection
- Affiliations
-
- 1Department of Pediatrics, Gyeongsang National University School of Medicine, Institute of Health Science, Jinju, Korea. hsyoun@gnu.ac.kr
- 2Department of Pathology, Gyeongsang National University School of Medicine, Institute of Health Science, Jinju, Korea.
- 3Department of Microbiology, Gyeongsang National University School of Medicine, Institute of Health Science, Jinju, Korea.
Abstract
- PURPOSE
Commercial enzyme-linked immunosorbent assay (ELISA) kits have been considered less reliable for children than for adults. The aim of this study was to compare four ELISA kits and in-house immunoblotting based on the analysis of anti-H. pylori-IgG antibody reactivity.
METHODS
A total of 399 serum samples were collected at the GNU Hospital during 1998-1999. All sera were tested using ELISA and immunoblotting. Statistically significant differences were determined by the chi2 test.
RESULTS
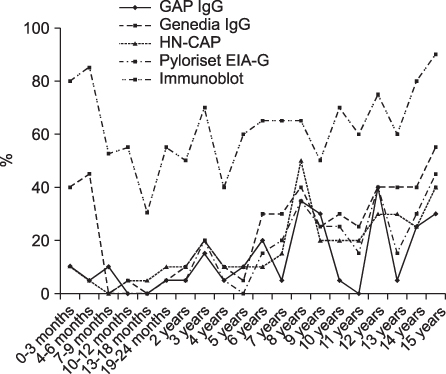
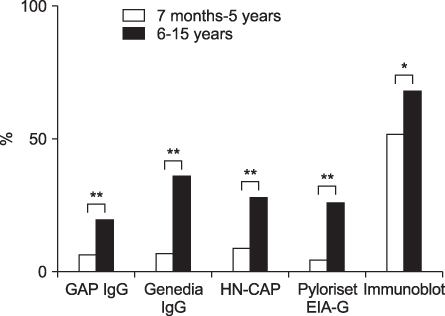
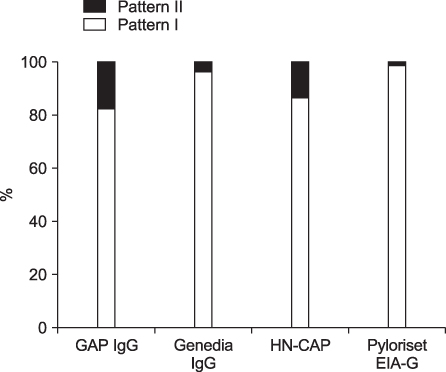
The overall seropositivity rates using GAP IgG, Genedia IgG, HM-CAP, Pyloriset EIA-G, and immunoblotting were 13.0%, 25.1%, 18.3%, 15.8%, and 62.9%, respectively. Immunoblotting showed a higher seropositivity rate than did all four ELISA kits in all age groups. Genedia IgG had the highest seropositivity among the ELISA kits. The seropositivity rate for children aged 13 to 18 months was lowest, and that of children aged 15 years was highest (90.0%). The seropositivity rate for children aged 7 months to 5 years was significantly lower than that for children aged 6 to 15 years among the four ELISA kits (p<0.0001) and immunoblotting (p=0.02).
CONCLUSION
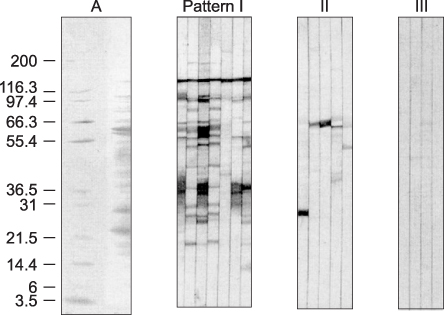
Immunoblotting is the most sensitive test for detection of anti-Helicobacter pylori IgG antibodies among the serological tests in this study. These results emphasize the need for standardization when commercial ELISA tests are used in different nations or in young age groups. Immunoblotting could be a suitable noninvasive assay for serodiagnosis and seroepidemiologic study of H. pylori infection in Korean children.
Keyword
MeSH Terms
Figure
Reference
-
1. Brown KE, Peura DA. Diagnosis of Helicobacter pylori infection. Gastroenterol Clin North Am. 1993. 22:105–115.2. Carpenter HA, Talley NJ. Gastroscopy is incomplete without biopsy: clinical relevance of distinguishing gastropathy from gastritis. Gastroenterology. 1995. 108:917–924.
Article3. Cutler AF, Havstead S, Ma CK, Blaser MJ, Perez-Perez GI, Schubert TT. Accuracy of invasive and non-invasive test to diagnose Helicobacter pylori infection. Gastroenterology. 1995. 109:136–141.
Article4. Leung Wk, Chan FKL, Falk MS, Suen R, Sung JJY. Comparison of two rapid whole-blood tests for Helicobacter pylori infection in Chinese patients. J Clin Microbiol. 1998. 36:3441–3442.
Article5. Khanna B, Cutler A, Israel NR, Perry M, Lastovica A, Fields PI, et al. Use caution with serologic testing for Helicobacter pylori infection in children. J Infect Dis. 1998. 178:460–465.
Article6. Raymond J, Sauvestre C, Kalach N, Bergeret M, Dupont C. Immunoblotting and serology for diagnosis of Helicobacter pylori infection in children. Pediatr Infect Dis J. 2000. 19:118–121.
Article7. Drumm B, Koletzko S, Oderda G. Helicobacter pylori infection in children: a consensus statement. J Pediatr Gastroenterol Nutr. 2000. 30:207–213.8. Kindermann A, Konstantopoulos N, Lehn N, Demmelmair H, Koletzko S. Evaluation of two commercial enzyme immunoassays, testing immunoglobulin G (IgG) and IgA responses, for diagnosis of Helicobacter pylori infection in children. J Clin Microbiol. 2001. 39:3591–3596.
Article9. Raymond J, Sauvestre C, Kalach N, Bergeret M, Dupont C. Immunoblotting and serology for diagnosis of Helicobacter pylori infection in children. Pediatr Infect Dis J. 2000. 19:118–121.
Article10. Youn HS, Baik SC, Lee WK, Cho MJ, Ryou HH, Choi HJ, et al. Serodiagnosis of Helicobacter pylori infection. J Bacteriol Virol. 1990. 25:463–474.11. Best LM, Veldhuyzen van Zanten SJ, Sherman PM, Berganson GS. Serological detection of Helicobacter pylori antibodies in children and their parents. J Clin Microbiol. 1994. 32:1193–1196.
Article12. Leung WK, Ng EK, Chan FK, Chung SC, Sung JJ. Evaluation of three commercial enzyme-linked immunosorbent assay kits for diagnosis of Helicobacter pylori in Chinese patients. Diagn Microbiol Infect Dis. 1999. 34:13–17.
Article13. Lee KL, Lim CY, Lee DH, Yoon JH, Lee HJ, Chung HC, et al. Evaluation of CtuickVue H. pylori test and IgG ELISA test in Helicobacter pylori infection. Korean J Gastroenterol. 1997. 29:35–40.14. Hong SP, Park HJ, Park IS, Lee KW, Kim HG. Serological diagnosis of Helicobacter pylori infection: comparison of diagnostic values between HM-CAP (EPI) test and GAP (Bio-Rad) test. Korean J Gastroenterol. 1995. 27:167–173.15. Kim JH, Kim HY, Kim NY, Kim SW, Kim JG, Kim JJ, et al. Seroepidemiological study of Helicobacter pylori infection in asymptomatic people in South Korea. J Gastroenterol Hepatol. 2001. 16:969–975.
Article16. Jung IS, Kim SW, Ko JS, Kim NY, Kim JG, Kim JH, et al. Accuracy of Genedia™ H. pylori ELISA for the diagnosis of Helicobacter pylori infection in Korean population. Korean J Med. 2001. 61:17–23.17. Andersen LP, Wewer AV, Christiansen KM, Tvede M, Hansen JP, Henriksen FW, et al. The humoral immune response to Helicobacter pylori infection in children with recurrent abdominal pain. APMIS. 1994. 102:457–464.
Article18. Yamaoka Y, Kodama T, Gutierrez O, Kim JG, Kashima K, Graham DY. Relationship between Helicobacter pylori iceA, cagA, and vacA status and clinical outcome: studies in four different countries. J Clin Microbiol. 1999. 37:2274–2279.
Article19. Yamaoka Y, Kodama T, Graham DY, Kashima K. Comparison of four serological tests to determine the CagA or VacA status of Helicobacter pylori strains. J Clin Microbiol. 1998. 36:3433–3434.
Article20. Ko JS, Kim KM, Oh YL, Seo JK. cagA, vacA, and iceA genotypes of Helicobacter pylori in Korean children. Pediatr Int. 2008. 50:628–631.21. Hsu PI, Lai KH, Tseng HH, Liu YU, Yen MY, Lin CK, et al. Correlation of serum immunoglobulin G Helicobacter pylori antibody levels with histologic and endoscopic findings in patients with dyspepsia. J Clin Gastroenterol. 1997. 25:587–591.
Article22. Chen TS, Li FY, Chang FY, Lee SD. Immunoglobulin G antibody against Helicobacter pylori: clinical implications of levels found in serum. Clin Diagn Lab Immunol. 2002. 9:1044–1048.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Usefulness of Helicobacter pylori IgG Ab Assay: Comparison of Six Commercial Kits
- Evaluation of Four commercial Enzyme Immunoassay for Detection of Helicobacter pylori Infection
- A Comparison of Accuracy between A New Commercial ELISA Test, GenediaTM Test and Other Commercial ELISA Tests for Serological Diagnosis of Helicobacter pylori Infection in Korea
- Evaluation of Diagnostic Usefulness for Genedia(R) Helicobacter pylori ELISA
- Evaluation of QuickVue H.pylori Test and IgG ELISA Test in Helicobacter pylori Infection