Pediatr Allergy Respir Dis.
2012 Dec;22(4):390-396.
Comparative Analysis of Immunoreactivity between Individual Serum and Pooled Serum in Serum Screening
- Affiliations
-
- 1Samsung Biomedical Research Institute, Seoul, Korea.
- 2Environmental Health Center for Atopic Diseases, Seoul, Korea. kmaped@skku.edu
- 3Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
Abstract
- PURPOSE
Serum screening test to detect specific immunoglobulin E (IgE) is an important step for the assessment of potential allergenicity of genetically modified (GM) food. The purpose of this study was to evaluate the usefulness of pooled serum for serum screening instead of individual serum.
METHODS
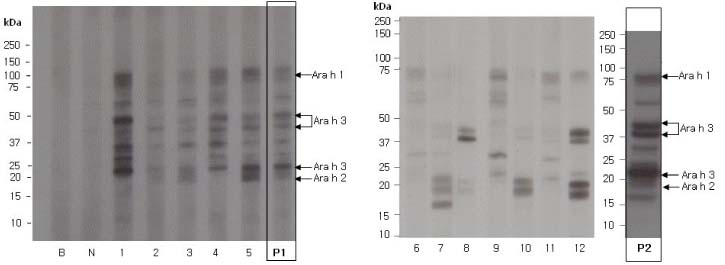
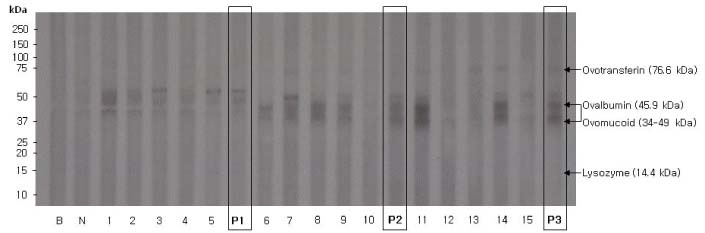
Children with allergic disease were recruited and those who were sensitized to peanut or egg white were selected to obtain their sera. Sensitization to these foods was determined when the level of specific IgE was over 0.35 kU/L by ImmunoCAP. The patients were divided into subgroups according to their level of specific IgE. Raw proteins were extracted and immunoblot analysis was performed to compare the immunoreactivity between individual serum and pooled serum.
RESULTS
Pooled serum from peanut-sensitized allergic children showed all the bands which were shown in immunoblot analysis by using individual serum and peanut protein extract. These findings were demonstrated both in pooled serum with low level of peanut-specific IgE and in those with high level of peanut-specific IgE. Likewise, there was no difference in the immunoreactivity between individual serum and pooled serum from egg white-sensitized allergic children.
CONCLUSION
Pooled serum can be used as an alternative to individual serum for the serum screening in the allergenicity assessment of GM food.
MeSH Terms
Figure
Reference
-
1. Metcalfe DD. Genetically modified crops and allergenicity. Nat Immunol. 2005. 6:857–860.
Article2. Taylor SL. Review of the development of methodology for evaluating the human allergenic potential of novel proteins. Mol Nutr Food Res. 2006. 50:604–609.
Article3. Goodman RE, Vieths S, Sampson HA, Hill D, Ebisawa M, Taylor SL, et al. Allergenicity assessment of genetically modified crops: what makes sense? Nat Biotechnol. 2008. 26:73–81.
Article4. Ladics GS. Current codex guidelines for assessment of potential protein allergenicity. Food Chem Toxicol. 2008. 46:Suppl 10. S20–S23.
Article5. Ladics GS, Selgrade MK. Identifying food proteins with allergenic potential: evolution of approaches to safety assessment and research to provide additional tools. Regul Toxicol Pharmacol. 2009. 54:3 Suppl. S2–S6.
Article6. Selgrade MK, Bowman CC, Ladics GS, Privalle L, Laessig SA. Safety assessment of biotechnology products for potential risk of food allergy: implications of new research. Toxicol Sci. 2009. 110:31–39.
Article7. Thomas K, MacIntosh S, Bannon G, Herouet-Guicheney C, Holsapple M, Ladics G, et al. Scientific advancement of novel protein allergenicity evaluation: an overview of work from the HESI Protein Allergenicity Technical Committee (2000-2008). Food Chem Toxicol. 2009. 47:1041–1050.
Article8. Liu Y, Zheng K, Chen M, Fu L, Du W, Shi Z. Study on detecting antibodies to Toxoplasma gondii in pooled serum of blood donors by Dot-IGSS. Southeast Asian J Trop Med Public Health. 2001. 32:558–561.9. Sadiq ST, Agranoff D. Pooling serum samples may lead to loss of potential biomarkers in SELDI-ToF MS proteomic profiling. Proteome Sci. 2008. 6:16.
Article10. Howanitz PJ, McBride JH, Kliewer KE, Rodgerson DO. Prevalence of antibodies to HTLV-III in quality-assurance sera. Clin Chem. 1986. 32:773–777.
Article11. Cahoon-Young B, Chandler A, Livermore T, Gaudino J, Benjamin R. Sensitivity and specificity of pooled versus individual sera in a human immunodeficiency virus antibody prevalence study. J Clin Microbiol. 1989. 27:1893–1895.
Article12. Liu P, Shi ZX, Zhang YC, Xu ZC, Shu HS, Zhang XY. A prospective study of a serum-pooling strategy in screening blood donors for antibody to hepatitis C virus. Transfusion. 1997. 37:732–736.
Article13. Gizzarelli F, Corinti S, Barletta B, Iacovacci P, Brunetto B, Butteroni C, et al. Evaluation of allergenicity of genetically modified soybean protein extract in a murine model of oral allergen-specific sensitization. Clin Exp Allergy. 2006. 36:238–248.
Article14. Taylor SL, Hefle SL. Genetically engineered foods: implications for food allergy. Curr Opin Allergy Clin Immunol. 2002. 2:249–252.
Article15. Prescott VE, Hogan SP. Genetically modified plants and food hypersensitivity diseases: usage and implications of experimental models for risk assessment. Pharmacol Ther. 2006. 111:374–383.
Article16. Batista R, Nunes B, Carmo M, Cardoso C, Jose HS, de Almeida AB, et al. Lack of detectable allergenicity of transgenic maize and soya samples. J Allergy Clin Immunol. 2005. 116:403–410.
Article17. Hoff M, Son DY, Gubesch M, Ahn K, Lee SI, Vieths S, et al. Serum testing of genetically modified soybeans with special emphasis on potential allergenicity of the heterologous protein CP4 EPSPS. Mol Nutr Food Res. 2007. 51:946–955.
Article18. Yum HY, Lee SY, Lee KE, Sohn MH, Kim KE. Genetically modified and wild soybeans: an immunologic comparison. Allergy Asthma Proc. 2005. 26:210–216.19. Kim SH, Kim HM, Ye YM, Kim SH, Nahm DH, Park HS, et al. Evaluating the allergic risk of genetically modified soybean. Yonsei Med J. 2006. 47:505–512.
Article20. Kim HH. Clinical significance of exhaled nitric oxide concentration in childhood asthma. Pediatr Allergy Respir Dis. 2009. 19:205–208.21. Kim JH, Seo YJ, Kim JY, Han YS, Lee KS, Kim SA, et al. Allergenicity assessment of cry proteins in insect-resistant genetically modified Maize Bt11, MON810, and MON863. Food Sci Biotechnol. 2009. 18:1273–1278.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Determination of Serum Pre-beta-Lipoprotein in Normal Individual by Electrophoresis on Cellulose Acetate Membrane
- Serum Uric Acid Levels In Korean Adult Population And Their Correlates
- Prenatal maternal serum screening for fetal aneuploidy
- Efficacy of Serum CA 125 and Ca 15-3 Levels in Appraently Healthy Women for Ovarian Cancer Screening
- A Study on the Serum Lipoperoxide Level in Normal Koreans