Obstet Gynecol Sci.
2014 Nov;57(6):492-500. 10.5468/ogs.2014.57.6.492.
Human papillomavirus 18 as a poor prognostic factor in stage I-IIA cervical cancer following primary surgical treatment
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Gachon University Gil Medical Center, Incheon, Korea. miracle627@gilhospital.com
- KMID: 2314016
- DOI: http://doi.org/10.5468/ogs.2014.57.6.492
Abstract
OBJECTIVE
This study evaluates the effect of the specific human papillomavirus (HPV) genotype as a prognostic factor in stage I-IIA cervical cancer patients following primary surgical treatment.
METHODS
The medical records of 116 cervical cancer patients treated with primary surgical treatment were reviewed. The HPV genotypes were categorized into following groups: negative and unclassified, HPV 16, HPV 18, and other high risk (HPV 31, 33, 35, 45, 51, 52, 56, and 58).
RESULTS
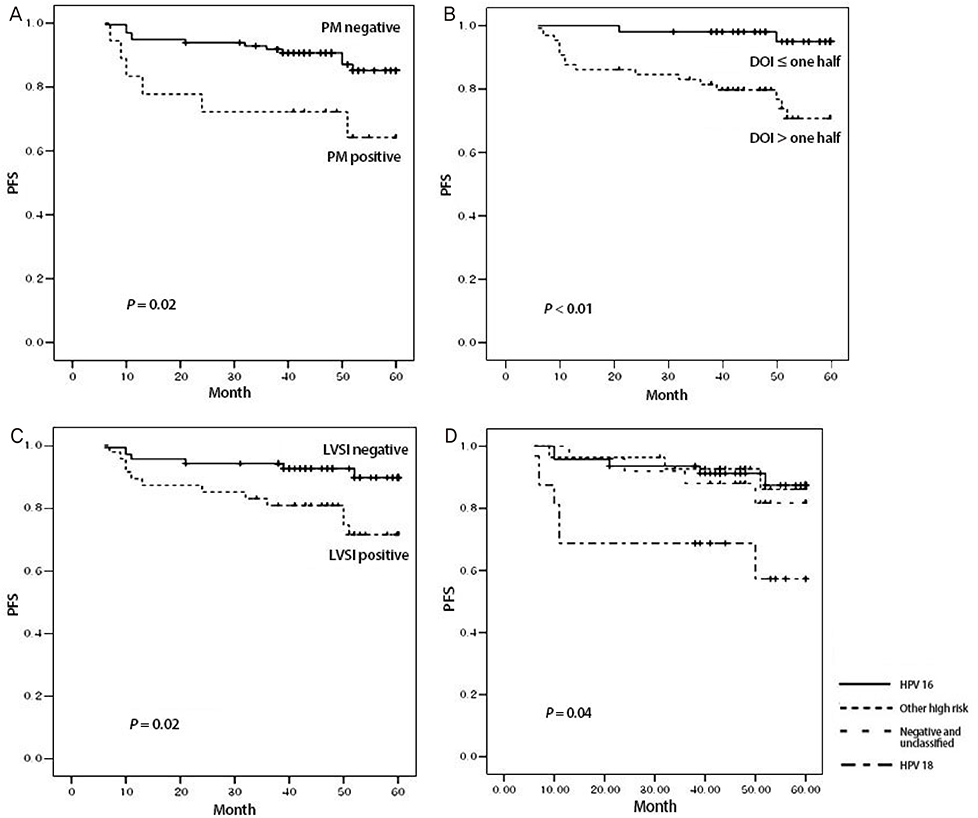
Among the HPV genotypes, HPV 16 predominated (40.52%), followed by intermediate risk and unclassified (25%), HPV 18, 45, and 56 (17.24%) and negative (17.24%). In univariate analysis, HPV genotypes (P=0.03), parametrial spread (P=0.02), depth of invasion (DOI) (P<0.01) and lymph-vascular space invasion (P=0.02) were significantly associated with progression free survival (PFS). In multivariate analysis, HPV 18 (hazard ratio [HR], 5.2; 95% confidence interval [CI], 1.29 to 20.90; P=0.02) and > or =one half of DOI (HR, 5.4; 95% CI, 1.08 to 27.31; P=0.04) were significantly associated with PFS. HPV genotypes are not significantly associated with overall survival.
CONCLUSION
HPV 18 was a poor prognostic factor for the PFS in stage I-IIA cervical cancer patients following primary surgical treatment. Careful long-term observation and regular exams are recommended for cervical cancer patients with HPV 18 compared to those with other HPV genotypes.
MeSH Terms
Figure
Reference
-
1. Ferlay J. International Agency for Research on Cancer. GLOBOCAN 2008: cancer incidence, mortality, and prevalence worldwide. Lyon: IARD Press;2008.2. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010; 60:277–300.3. Kim YT. Current status of cervical cancer and HPV infection in Korea. J Gynecol Oncol. 2009; 20:1–7.4. Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003; 348:518–527.5. Van Muyden RC, ter Harmsel BW, Smedts FM, Hermans J, Kuijpers JC, Raikhlin NT, et al. Detection and typing of human papillomavirus in cervical carcinomas in Russian women: a prognostic study. Cancer. 1999; 85:2011–2016.6. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999; 189:12–19.7. Zur Hausen H. Papillomaviruses causing cancer: evasion from host-cell control in early events in carcinogenesis. J Natl Cancer Inst. 2000; 92:690–698.8. Rose BR, Thompson CH, Simpson JM, Jarrett CS, Elliott PM, Tattersall MH, et al. Human papillomavirus deoxyribonucleic acid as a prognostic indicator in early-stage cervical cancer: a possible role for type 18. Am J Obstet Gynecol. 1995; 173:1461–1468.9. Nakagawa S, Yoshikawa H, Onda T, Kawana T, Iwamoto A, Taketani Y. Type of human papillomavirus is related to clinical features of cervical carcinoma. Cancer. 1996; 78:1935–1941.10. Burger RA, Monk BJ, Kurosaki T, Anton-Culver H, Vasilev SA, Berman ML, et al. Human papillomavirus type 18: association with poor prognosis in early stage cervical cancer. J Natl Cancer Inst. 1996; 88:1361–1368.11. Lombard I, Vincent-Salomon A, Validire P, Zafrani B, de la Rochefordiere A, Clough K, et al. Human papillomavirus genotype as a major determinant of the course of cervical cancer. J Clin Oncol. 1998; 16:2613–2619.12. Schwartz SM, Daling JR, Shera KA, Madeleine MM, McKnight B, Galloway DA, et al. Human papillomavirus and prognosis of invasive cervical cancer: a population-based study. J Clin Oncol. 2001; 19:1906–1915.13. Im SS, Wilczynski SP, Burger RA, Monk BJ. Early stage cervical cancers containing human papillomavirus type 18 DNA have more nodal metastasis and deeper stromal invasion. Clin Cancer Res. 2003; 9:4145–4150.14. Viladiu P, Bosch FX, Castellsague X, Munoz N, Escriba JM, Hamsikova E, et al. Human papillomavirus DNA and antibodies to human papillomaviruses 16 E2, L2, and E7 peptides as predictors of survival in patients with squamous cell cervical cancer. J Clin Oncol. 1997; 15:610–619.15. Huang LW, Chao SL, Hwang JL. Human papillomavirus-31-related types predict better survival in cervical carcinoma. Cancer. 2004; 100:327–334.16. Lai HC, Sun CA, Yu MH, Chen HJ, Liu HS, Chu TY. Favorable clinical outcome of cervical cancers infected with human papilloma virus type 58 and related types. Int J Cancer. 1999; 84:553–557.17. Van Bommel PF, van den Brule AJ, Helmerhorst TJ, Gallee MP, Gaarenstroom KN, Walboomers JM, et al. HPV DNA presence and HPV genotypes as prognostic factors in low-stage squamous cell cervical cancer. Gynecol Oncol. 1993; 48:333–337.18. Fule T, Csapo Z, Mathe M, Tatrai P, Laszlo V, Papp Z, et al. Prognostic significance of high-risk HPV status in advanced cervical cancers and pelvic lymph nodes. Gynecol Oncol. 2006; 100:570–578.19. Rustin GJ, Quinn M, Thigpen T, du Bois A, Pujade-Lauraine E, Jakobsen A, et al. Re: New guidelines to evaluate the response to treatment in solid tumors (ovarian cancer). J Natl Cancer Inst. 2004; 96:487–488.20. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–247.21. Pilch H, Günzel S, Schaffer U, Tanner B, Brockerhoff P, Maeurer M, et al. The presence of HPV DNA in cervical cancer: correlation with clinico-pathologic parameters and prognostic significance: 10 years experience at the Department of Obstetrics and Gynecology of the Mainz University. Int J Gynecol Cancer. 2001; 11:39–48.22. Bachtiary B, Obermair A, Dreier B, Birner P, Breitenecker G, Knocke TH, et al. Impact of multiple HPV infection on response to treatment and survival in patients receiving radical radiotherapy for cervical cancer. Int J Cancer. 2002; 102:237–243.23. Kang WD, Kim CH, Cho MK, Kim JW, Cho HY, Kim YH, et al. HPV-18 is a poor prognostic factor, unlike the HPV viral load, in patients with stage IB-IIA cervical cancer undergoing radical hysterectomy. Gynecol Oncol. 2011; 121:546–550.24. Peters WA 3rd, Liu PY, Barrett RJ 2nd, Stock RJ, Monk BJ, Berek JS, et al. Concurrent chemotherapy and pelvic radiation therapy compared with pelvic radiation therapy alone as adjuvant therapy after radical surgery in high-risk early-stage cancer of the cervix. J Clin Oncol. 2000; 18:1606–1613.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Significance of Human Papillomavirus Types 16 and 18 in Invasive Cervical Cancer
- Is human papillomavirus genotype an influencing factor on radiotherapy outcome? Ambiguity caused by an association of HPV 18 genotype and adenocarcinoma histology
- The Application of Human Papillomavirus Testing to Cervical Cancer Screening
- Clinical Significance of Human Papillomavirus Infection and Epidermal Growth Factor Receptor in Cervical Carcinoma
- Low initial human papillomavirus viral load may indicate worse prognosis in patients with cervical carcinoma treated with surgery