Low initial human papillomavirus viral load may indicate worse prognosis in patients with cervical carcinoma treated with surgery
- Affiliations
-
- 1State Key Laboratory of Oncology in South China, Sun Yat-sen University Cancer Center, Guangzhou, China. liujh@mail.sysu.edu.cn
- 2Department of Gynecologic Oncology, Sun Yat-sen University Cancer Center, Guangzhou, China.
- 3Department of Gynecology and Obstetrics, Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China.
- KMID: 2160793
- DOI: http://doi.org/10.3802/jgo.2015.26.2.111
Abstract
OBJECTIVE
To evaluate the prognostic implication of human papillomavirus (HPV) viral load in cervical cancer patients who underwent radical hysterectomy.
METHODS
We conducted a retrospective review of patients with stage IA2 through stage IIIA cervical carcinoma who underwent radical hysterectomy at Sun Yat-sen University Cancer Center between January 2005 and December 2009. Patients who had undergone preoperative hybrid capture 2 testing to detect HPV DNA were included. A total of 346 patients positive for HPV DNA were enrolled and stratified into two groups according to the median HPV viral load.
RESULTS
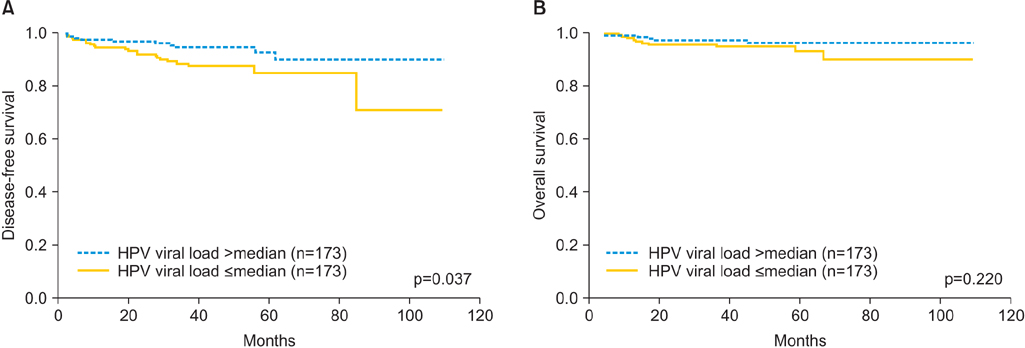
HPV viral load was significantly correlated with lymphovascular space invasion (p=0.026) and deep stromal invasion (p=0.024). However, other factors, such as age, stage, histologic grade, histologic type, lymph node metastasis, and tumor size, were not significantly associated with viral load. Low HPV viral load was correlated with poor disease-free survival in univariate analysis (p=0.037) and multivariate analysis (p=0.027). There was no significant difference in overall survival with regard to initial HPV viral load.
CONCLUSION
Low initial HPV viral load may be a poor prognostic factor for cervical cancer patients who have undergone radical hysterectomy.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Carcinoma, Squamous Cell/*diagnosis/surgery/virology
Female
Humans
Middle Aged
Papillomaviridae/*isolation & purification
Papillomavirus Infections/complications/diagnosis/surgery/virology
Prognosis
Retrospective Studies
Treatment Outcome
Uterine Cervical Neoplasms/*diagnosis/surgery/virology
*Viral Load
Young Adult
Figure
Cited by 2 articles
-
Human papillomavirus (HPV) DNA detection in uterine cervix cancer after radiation indicating recurrence: a systematic review and meta-analysis
Sasidharanpillai Sabeena, Santhosh Kuriakose, Binesh Damodaran, Nagaraja Ravishankar, Govindakarnavar Arunkumar
J Gynecol Oncol. 2020;31(2):. doi: 10.3802/jgo.2020.31.e20.The Impact of High-Risk HPV Genotypes Other Than HPV 16/18 on the Natural Course of Abnormal Cervical Cytology: A Korean HPV Cohort Study
Kyeong A So, Mi Jung Kim, Ki-Heon Lee, In-Ho Lee, Mi Kyung Kim, Yoo Kyung Lee, Chang-Sun Hwang, Mi Seon Jeong, Mee-Kyung Kee, Chun Kang, Chi Heum Cho, Seok Mo Kim, Sung Ran Hong, Ki Tae Kim, Won-Chul Lee, Jong Sup Park, Tae Jin Kim
Cancer Res Treat. 2016;48(4):1313-1320. doi: 10.4143/crt.2016.013.
Reference
-
1. Sandri MT, Lentati P, Benini E, Dell'Orto P, Zorzino L, Carozzi FM, et al. Comparison of the Digene HC2 assay and the Roche AMPLICOR human papillomavirus (HPV) test for detection of high-risk HPV genotypes in cervical samples. J Clin Microbiol. 2006; 44:2141–2146.2. Origoni M, Carminati G, Rolla S, Clementi M, Sideri M, Sandri MT, et al. Human papillomavirus viral load expressed as relative light units (RLU) correlates with the presence and grade of preneoplastic lesions of the uterine cervix in atypical squamous cells of undetermined significance (ASCUS) cytology. Eur J Clin Microbiol Infect Dis. 2012; 31:2401–2406.3. Park JY, Lee KH, Dong SM, Kang S, Park SY, Seo SS. The association of pre-conization high-risk HPV load and the persistence of HPV infection and persistence/recurrence of cervical intraepithelial neoplasia after conization. Gynecol Oncol. 2008; 108:549–554.4. Wu Y, Chen Y, Li L, Yu G, Zhang Y, He Y. Associations of high-risk HPV types and viral load with cervical cancer in China. J Clin Virol. 2006; 35:264–269.5. Ho CM, Cheng WF, Chu TY, Chen CA, Chuang MH, Chang SF, et al. Human papillomaviral load changes in low-grade squamous intraepithelial lesions of the uterine cervix. Br J Cancer. 2006; 95:1384–1389.6. Hernandez-Hernandez DM, Ornelas-Bernal L, Guido-Jimenez M, Apresa-Garcia T, Alvarado-Cabrero I, Salcedo-Vargas M, et al. Association between high-risk human papillomavirus DNA load and precursor lesions of cervical cancer in Mexican women. Gynecol Oncol. 2003; 90:310–317.7. Dalstein V, Riethmuller D, Pretet JL, Le Bail Carval K, Sautiere JL, Carbillet JP, et al. Persistence and load of high-risk HPV are predictors for development of high-grade cervical lesions: a longitudinal French cohort study. Int J Cancer. 2003; 106:396–403.8. Kim JY, Park S, Nam BH, Roh JW, Lee CH, Kim YH, et al. Low initial human papilloma viral load implicates worse prognosis in patients with uterine cervical cancer treated with radiotherapy. J Clin Oncol. 2009; 27:5088–5093.9. Song YJ, Kim JY, Lee SK, Lim HS, Lim MC, Seo SS, et al. Persistent human papillomavirus DNA is associated with local recurrence after radiotherapy of uterine cervical cancer. Int J Cancer. 2011; 129:896–902.10. Datta NR, Kumar P, Singh S, Gupta D, Srivastava A, Dhole TN. Does pretreatment human papillomavirus (HPV) titers predict radiation response and survival outcomes in cancer cervix? A pilot study. Gynecol Oncol. 2006; 103:100–105.11. Doorbar J. Papillomavirus life cycle organization and biomarker selection. Dis Markers. 2007; 23:297–313.12. Ganguly N, Parihar SP. Human papillomavirus E6 and E7 oncoproteins as risk factors for tumorigenesis. J Biosci. 2009; 34:113–123.13. Finzer P, Aguilar-Lemarroy A, Rosl F. The role of human papillomavirus oncoproteins E6 and E7 in apoptosis. Cancer Lett. 2002; 188:15–24.14. Jeon S, Lambert PF. Integration of human papillomavirus type 16 DNA into the human genome leads to increased stability of E6 and E7 mRNAs: implications for cervical carcinogenesis. Proc Natl Acad Sci U S A. 1995; 92:1654–1658.15. Smotkin D, Wettstein FO. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci U S A. 1986; 83:4680–4684.16. Androphy EJ, Hubbert NL, Schiller JT, Lowy DR. Identification of the HPV-16 E6 protein from transformed mouse cells and human cervical carcinoma cell lines. EMBO J. 1987; 6:989–992.17. Banks L, Spence P, Androphy E, Hubbert N, Matlashewski G, Murray A, et al. Identification of human papillomavirus type 18 E6 polypeptide in cells derived from human cervical carcinomas. J Gen Virol. 1987; 68(Pt 5):1351–1359.18. Hampson L, El Hady ES, Moore JV, Kitchener H, Hampson IN. The HPV16 E6 and E7 proteins and the radiation resistance of cervical carcinoma. FASEB J. 2001; 15:1445–1447.19. Zheng Y, Zhang J, Rao Z. Ribozyme targeting HPV16 E6E7 transcripts in cervical cancer cells suppresses cell growth and sensitizes cells to chemotherapy and radiotherapy. Cancer Biol Ther. 2004; 3:1129–1134.20. Waggoner SE. Cervical cancer. Lancet. 2003; 361:2217–2225.21. Benedet JL, Bender H, Jones H 3rd, Ngan HY, Pecorelli S. FIGO staging classifications and clinical practice guidelines in the management of gynecologic cancers. FIGO Committee on Gynecologic Oncology. Int J Gynaecol Obstet. 2000; 70:209–262.22. Landoni F, Maneo A, Colombo A, Placa F, Milani R, Perego P, et al. Randomised study of radical surgery versus radiotherapy for stage Ib-IIa cervical cancer. Lancet. 1997; 350:535–540.23. Delgado G, Bundy B, Zaino R, Sevin BU, Creasman WT, Major F. Prospective surgical-pathological study of disease-free interval in patients with stage IB squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. Gynecol Oncol. 1990; 38:352–357.24. Kim YM, Park JY, Lee KM, Kong TW, Yoo SC, Kim WY, et al. Does pretreatment HPV viral load correlate with prognosis in patients with early stage cervical carcinoma? J Gynecol Oncol. 2008; 19:113–116.25. Hubbard RA. Human papillomavirus testing methods. Arch Pathol Lab Med. 2003; 127:940–945.26. Bolick DR, Bolick RE, Coates F, Daniels CM, Juretich MB, Lin KK, et al. Laboratory implementation of human papillomavirus testing. Arch Pathol Lab Med. 2003; 127:984–990.27. Castle PE, Lorincz AT, Mielzynska-Lohnas I, Scott DR, Glass AG, Sherman ME, et al. Results of human papillomavirus DNA testing with the hybrid capture 2 assay are reproducible. J Clin Microbiol. 2002; 40:1088–1090.28. Castle PE, Wheeler CM, Solomon D, Schiffman M, Peyton CL. ALTS Group. Interlaboratory reliability of hybrid capture 2. Am J Clin Pathol. 2004; 122:238–245.29. Clavel C, Masure M, Levert M, Putaud I, Mangeonjean C, Lorenzato M, et al. Human papillomavirus detection by the hybrid capture II assay: a reliable test to select women with normal cervical smears at risk for developing cervical lesions. Diagn Mol Pathol. 2000; 9:145–150.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Does pretreatment HPV viral load correlate with prognosis in patients with early stage cervical carcinoma?
- The utility of the human papillomavirus DNA load for the diagnosis and prediction of persistent vaginal intraepithelial neoplasia
- The association of the cervical intraepithelial neoplasia and human papillomavirus viral load
- Physical Status and Viral load in Women with Positive Human Papillomavirus (HPV) Infection in Uterine Cervix
- The significance of Human Papillomavirus viral load by using hybrid capture II assay in diagnosis of CIN II or above in women with PAP smear showing only ASCUS/LSIL