Nutr Res Pract.
2011 Jun;5(3):205-213.
Exercise training and selenium or a combined treatment ameliorates aberrant expression of glucose and lactate metabolic proteins in skeletal muscle in a rodent model of diabetes
- Affiliations
-
- 1Exercise Biochemistry Laboratory, Korea National Sport University, 88-15 Oryun-dong, Songpa-gu, Seoul 138-763, Korea. chojy86@knsu.ac.kr
- 2Department of Sport Informatics, University of Seoul, Seoul 130-743, Korea.
- 3Depament of Physical Education, Keimyung University, Daegu 704-701, Korea.
- 4Department of Anatomy and Cell Biology, Collage of Medicine, Hanyang University, Seoul 133-791, Korea.
Abstract
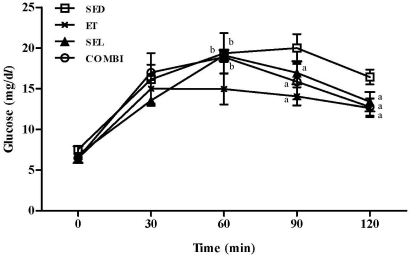
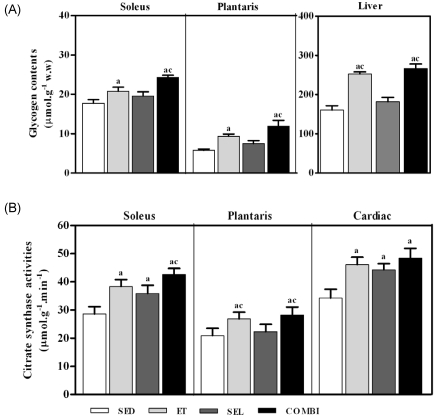
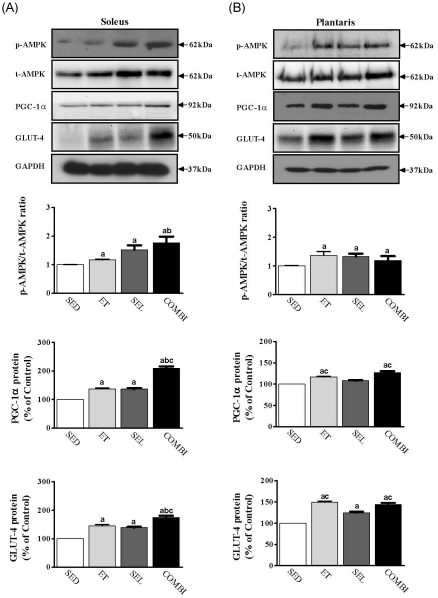
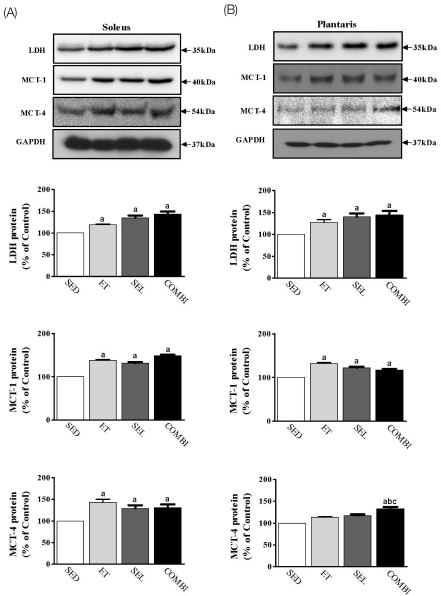
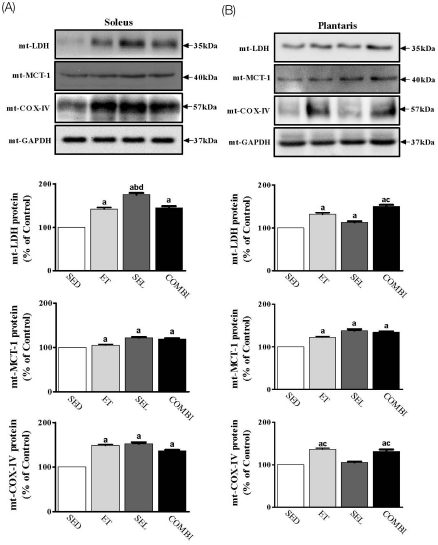
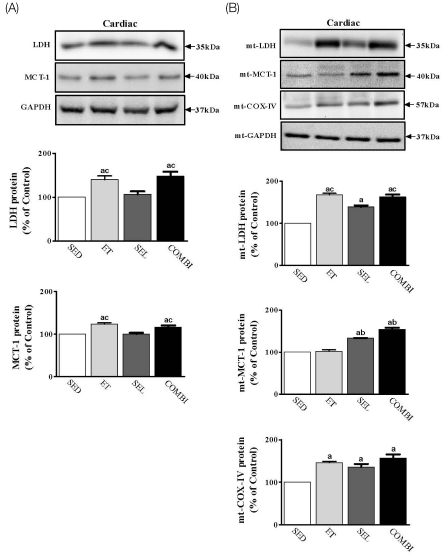
- Exercise training (ET) and selenium (SEL) were evaluated either individually or in combination (COMBI) for their effects on expression of glucose (AMPK, PGC-1alpha, GLUT-4) and lactate metabolic proteins (LDH, MCT-1, MCT-4, COX-IV) in heart and skeletal muscles in a rodent model (Goto-Kakisaki, GK) of diabetes. Forty GK rats either remained sedentary (SED), performed ET, received SEL, (5 micromol/kg body wt(-1)/day(-1)) or underwent both ET and SEL treatment for 6 wk. ET alone, SEL alone, or COMBI resulted in a significant lowering of lactate, glucose, and insulin levels as well as a reduction in HOMA-IR and AUC for glucose relative to SED. Additionally, ET alone, SEL alone, or COMBI increased glycogen content and citrate synthase (CS) activities in liver and muscles. However, their effects on glycogen content and CS activity were tissue-specific. In particular, ET alone, SEL alone, or COMBI induced upregulation of glucose (AMPK, PGC-1alpha, GLUT-4) and lactate (LDH, MCT-1, MCT-4, COX-IV) metabolic proteins relative to SED. However, their effects on glucose and lactate metabolic proteins also appeared to be tissue-specific. It seemed that glucose and lactate metabolic protein expression was not further enhanced with COMBI compared to that of ET alone or SEL alone. These data suggest that ET alone or SEL alone or COMBI represent a practical strategy for ameliorating aberrant expression of glucose and lactate metabolic proteins in diabetic GK rats.
Keyword
MeSH Terms
Figure
Reference
-
1. Almind K, Doria A, Kahn CR. Putting the genes for type II diabetes on the map. Nat Med. 2001; 7:277–279. PMID: 11231616.
Article2. Brown JB, Pedula K, Barzilay J, Herson MK, Latare P. Lactic acidosis rates in type 2 diabetes. Diabetes Care. 1998; 21:1659–1663. PMID: 9773726.
Article3. Song XM, Kawano Y, Krook A, Ryder JW, Efendic S, Roth RA, Wallberg-Henriksson H, Zierath JR. Muscle fiber type-specific defects in insulin signal transduction to glucose transport in diabetic GK rats. Diabetes. 1999; 48:664–670. PMID: 10078575.
Article4. Enoki T, Yoshida Y, Hatta H, Bonen A. Exercise training alleviates MCT1 and MCT4 reductions in heart and skeletal muscles of STZ-induced diabetic rats. J Appl Physiol. 2003; 94:2433–2438. PMID: 12611763.5. Metz L, Vermaelen M, Lambert K, Broca C, Sirvent P, Raynaud E, Mercier J. Endurance training increases lactate transport in male Zucker fa/fa rats. Biochem Biophys Res Commun. 2005; 331:1338–1345. PMID: 15883022.
Article6. Sriwijitkamol A, Coletta DK, Wajcberg E, Balbontin GB, Reyna SM, Barrientes J, Eagan PA, Jenkinson CP, Cersosimo E, DeFronzo RA, Sakamoto K, Musi N. Effect of acute exercise on AMPK signaling in skeletal muscle of subjects with type 2 diabetes: a time-course and dose-response study. Diabetes. 2007; 56:836–848. PMID: 17327455.7. Müller AS, Most E, Pallauf J. Effects of a supranutritional dose of selenate compared with selenite on insulin sensitivity in type II diabetic dbdb mice. J Anim Physiol Anim Nutr (Berl). 2005; 89:94–104. PMID: 15787978.8. Ostergård T, Nyholm B, Hansen TK, Rasmussen LM, Ingerslev J, Sørensen KE, Bøtker HE, Saltin B, Schmitz O. Endothelial function and biochemical vascular markers in first-degree relatives of type 2 diabetic patients: the effect of exercise training. Metabolism. 2006; 55:1508–1515. PMID: 17046554.
Article9. Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1alpha gene in human skeletal muscle. J Physiol. 2003; 546:851–858. PMID: 12563009.10. Arteel GE, Sies H. The biochemistry of selenium and the glutathione system. Environ Toxicol Pharmacol. 2001; 10:153–158.
Article11. Mckenzie RC, Arthur JR, Miller SM, Rafferty TS, Beckett GJ. Calder PC, Field CJ, Gill HS, editors. Selenium and immune function. Nutrition and immune function. 2002. Wallingford: CABI Publishing;p. 229–250.12. Faure P, Ramon O, Favier A, Halimi S. Selenium supplementation decreases nuclear factor-kappa B activity in peripheral blood mononuclear cells from type 2 diabetic patients. Eur J Clin Invest. 2004; 34:475–481. PMID: 15255784.
Article13. Diplock AT. Antioxidant nutrients and disease prevention: an overview. Am J Clin Nutr. 1991; 53(1 Suppl):189S–193S. PMID: 1985386.14. MacFarquhar JK, Broussard DL, Melstrom P, Hutchinson R, Wolkin A, Martin C, Burk RF, Dunn JR, Green AL, Hammond R, Schaffner W, Jones TF. Acute selenium toxicity associated with a dietary supplement. Arch Intern Med. 2010; 170:256–261. PMID: 20142570.
Article15. Bo S, Lezo A, Menato G, Gallo ML, Bardelli C, Signorile A, Berutti C, Massobrio M, Pagano GF. Gestational hyperglycemia, zinc, selenium, and antioxidant vitamins. Nutrition. 2005; 21:186–191. PMID: 15723747.
Article16. Ostenson CG, Efendic S. Islet gene expression and function in type 2 diabetes; studies in the Goto-Kakizaki rat and humans. Diabetes Obes Metab. 2007; 9(Suppl 2):180–186. PMID: 17919192.
Article17. American Physiological Society Operational Guide Revision. Guiding Principles for the Care and Use of Animal in the Recommendations for the Declaration of Helsinki 1964. 2002. The American Physiological Society;p. 297–306.18. Hwang D, Seo S, Kim Y, Kim C, Shim S, Jee S, Lee S, Jang M, Kim M, Yim S, Lee SK, Kang B, Jang I, Cho J. Selenium acts as an insulin-like molecule for the down-regulation of diabetic symptoms via endoplasmic reticulum stress and insulin signalling proteins in diabetes-induced non-obese diabetic mice. J Biosci. 2007; 32:723–735. PMID: 17762145.
Article19. Hamamoto Y, Tsuura Y, Fujimoto S, Nagata M, Takeda T, Mukai E, Fujita J, Yamada Y, Seino Y. Recovery of function and mass of endogenous beta-cells in streptozotocin-induced diabetic rats treated with islet transplantation. Biochem Biophys Res Commun. 2001; 287:104–109. PMID: 11549260.20. Lowry OH, Passonneau JV. A flexible system of enzymatic analysis. 1972. New York: Academic Press;p. 147–217.21. Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T, Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun. 2002; 296:350–354. PMID: 12163024.
Article22. Terada S, Tabata I. Effects of acute bouts of running and swimming exercise on PGC-1alpha protein expression in rat epitrochlearis and soleus muscle. Am J Physiol Endocrinol Metab. 2004; 286:E208–E216. PMID: 14570700.23. Ayaz M, Ozdemir S, Ugur M, Vassort G, Turan B. Effects of selenium on altered mechanical and electrical cardiac activities of diabetic rat. Arch Biochem Biophys. 2004; 426:83–90. PMID: 15130786.
Article24. Fürnsinn C, Englisch R, Ebner K, Nowotny P, Vogl C, Waldhäusl W. Insulin-like vs. non-insulin-like stimulation of glucose metabolism by vanadium, tungsten, and selenium compounds in rat muscle. Life Sci. 1996; 59:1989–2000. PMID: 8950298.
Article25. Tan MH, Bonen A, Garner JB, Belcastro AN. Physical training in diabetic rats: effect on glucose tolerance and serum lipids. J Appl Physiol. 1982; 52:1514–1518. PMID: 7107460.
Article26. Tancrède G, Rousseau-Migneron S, Nadeau A. Beneficial effects of physical training in rats with a mild streptozotocin-induced diabetes mellitus. Diabetes. 1982; 31:406–409. PMID: 6759257.
Article27. Hayashi T, Hirshman MF, Kurth EJ, Winder WW, Goodyear LJ. Evidence for 5' AMP-activated protein kinase mediation of the effect of muscle contraction on glucose transport. Diabetes. 1998; 47:1369–1373. PMID: 9703344.
Article28. Hardie DG, Sakamoto K. AMPK: a key sensor of fuel and energy status in skeletal muscle. Physiology (Bethesda). 2006; 21:48–60. PMID: 16443822.
Article29. Py G, Eydoux N, Perez-Martin A, Raynaud E, Brun JF, Préfaut C, Mercier J. Streptozotocin-induced diabetes decreases rat sarcolemmal lactate transport. Metabolism. 2001; 50:418–424. PMID: 11288036.
Article30. McCullagh KJ, Poole RC, Halestrap AP, O'Brien M, Bonen A. Role of the lactate transporter (MCT1) in skeletal muscles. Am J Physiol. 1996; 271:E143–E150. PMID: 8760092.
Article31. Hughes GM, Szegletes T, Nemcsók J. A comparison of blood composition in the common carp (Cyprinus carpio L.) using samples obtained from caudal vessels and dorsal aorta cannulae. Acta Biol Hung. 1997; 48:145–155. PMID: 9404538.32. Bonen A, Tonouchi M, Miskovic D, Heddle C, Heikkila JJ, Halestrap AP. Isoform-specific regulation of the lactate transporters MCT1 and MCT4 by contractile activity. Am J Physiol Endocrinol Metab. 2000; 279:E1131–E1138. PMID: 11052969.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Exercise on Glucose Metabolism
- Effect of Exercise Training on Insulin Sensitivity and Intracellular Glucose Metabolism in Skeletal Muscle of High Fat-fed Rats
- Effect of Exercise on Cardiovascular Disease in Patients with Diabetes Mellitus (Aerobic vs. Resistance)
- Pharmacokinetics and cytotoxic effect of selenium compounds in rodent cancer xenograft model for therapy experiment
- Exercise and Mitochondrial Remodeling in Skeletal Muscle in Type 2 Diabetes