Nutr Res Pract.
2009 Mar;3(1):3-8.
Blockade of vascular angiogenesis by Aspergillus usamii var. shirousamii-transformed Angelicae Gigantis Radix and Zizyphus jujuba
- Affiliations
-
- 1Department of Food and Nutrition and Korean Institute of Nutrition, Hallym University, 39 Hallymdaehak-gil, Chuncheon, Kangwon 200-702, Korea. yhkang@hallym.ac.kr
- 2Department of Food and Nutrition and Research Institute of Human Ecology, Seoul National University, 599 Gwanak-ro, Gwanak-gu, Seoul 151-742, Korea.
- 3Department of Environmental Engineering, Hanseo University, 360 Daegok-ri, Haemi-myun, Seosan, Chungnam 360-706, Korea.
- 4Research Institute, Bifido Inc., 688-1 Sangoan-ri, Hongchon, Kangwon 250-800, Korea.
Abstract
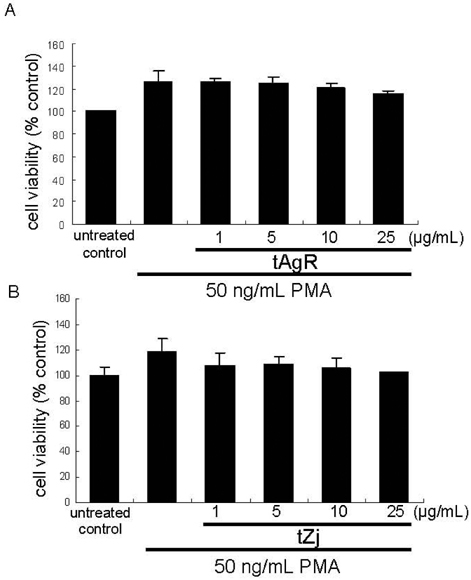
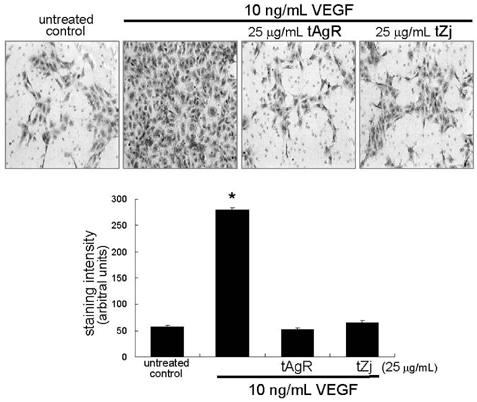
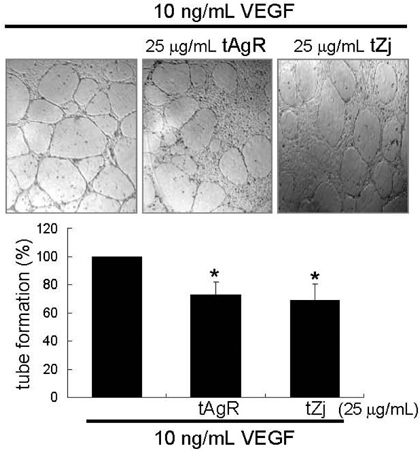
- The matrix metalloproteinases (MMP) play an important role in tumor invasion, angiogenesis and inflammatory tissue destruction. Increased expression of MMP was observed in benign tissue hyperplasia and in atherosclerotic lesions. Invasive cancer cells utilize MMP to degrade the extracellular matrix and vascular basement membrane during metastasis, where MMP-2 has been implicated in the development and dissemination of malignancies. The present study attempted to examine the antiangiogenic activity of the medicinal herbs of Aspergillus usamii var. shirousamii-transformed Angelicae Gigantis Radix and Zizyphus jujube (tAgR and tZj) with respect to MMP-2 production and endothelial motility in phorbol 12-myristate 13-acetate (PMA)- or VEGF-exposed human umbilical vein endothelial cells (HUVEC). Nontoxic tAgR and tZj substantially suppressed PMA-induced MMP-2 secretion. In addition, 25 microg/mL tAgR and tZj prevented vascular endothelial growth factor-stimulated endothelial cell transmigration and tube formation. The results reveal that tAgR and tZj dampened endothelial MMP-2 production leading to endothelial transmigration and tube formation. tAgR and tZj-mediated inhibition of endothelial MMP may boost a therapeutic efficacy during vascular angiogenesis.
Keyword
MeSH Terms
Figure
Reference
-
1. Battegay EJ. Angiogenesis: mechanistic insights, neovascular diseases, and therapeutic prospects. J Mol Med. 1995. 73:333–346.
Article2. Chi H, Kim DH, Ji GE. Transformation of ginsenosides Rb2 and Rc from Panax ginseng by food microorganisms. Biol Pharm Bull. 2005. 28:2102–2105.
Article3. Choi YJ, Jeong YJ, Lee YJ, Kwon HM, Kang YH. (-)Epigallocatechin gallate and quercetin enhance survival signaling in response to oxidant-induced human endothelial apoptosis. J Nutr. 2005. 135:707–713.
Article4. Choi YJ, Kang JS, Park JH, Lee YJ, Choi JS, Kang YH. Polyphenolic flavonoids differ in their antiapoptotic efficacy in hydrogen peroxide-treated human vascular endothelial cells. J Nutr. 2003. 133:985–991.
Article5. Eming SA, Brachvogel B, Odorisio T, Koch M. Regulation of angiogenesis: wound healing as a model. Prog Histochem Cytochem. 2007. 42:115–170.
Article6. Gao QT, Cheung JK, Choi RC, Cheung AW, Li J, Jiang ZY, Duan R, Zhao KJ, Ding AW, Dong TT, Tsim KW. A Chinese herbal decoction prepared from Radix Astragali and Radix Angelicae Sinensis induces the expression of erythropoietin in cultured Hep3B cells. Planta Med. 2008. 74:392–395.
Article7. Haines CJ, Lam PM, Chung TK, Cheng KF, Leung PC. A randomized, double-blind, placebo-controlled study of the effect of a Chinese herbal medicine preparation (Dang Gui Buxue Tang) on menopausal symptoms in Hong Kong Chinese women. Climacteric. 2008. 11:244–251.
Article8. Herath W, Reddy N, Khan IA. Microbial metabolism. The pyranocoumarin, decursin. Chem Pharm Bull (Tokyo). 2007. 55:1512–1513.9. Jiang JG, Huang XJ, Chen J, Lin QS. Comparison of the sedative and hypnotic effects of flavonoids, saponins, and polysaccharides extracted from Semen Ziziphus jujube. Nat Prod Res. 2007. 21:310–320.
Article10. Kargozaran H, Yuan SY, Breslin JW, Watson KD, Gaudreault N, Breen A, Wu MH. A role for endothelial-derived matrix metalloproteinase-2 in breast cancer cell transmigration across the endothelial-basement membrane barrier. Clin Exp Metastasis. 2007. 24:495–502.
Article11. Kil JS, Kim MG, Choi HM, Lim JP, Boo Y, Kim EH, Kim JB, Kim HK, Leem KH. Inhibitory effects of Angelicae Gigantis Radix on osteoclast formation. Phytother Res. 2008. 22:472–476.
Article12. Li J, Zhang YP, Kirsner RS. Angiogenesis in wound repair: Angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003. 60:107–114.
Article13. Liekens S, De Clercq E, Neyts J. Angiogenesis: Regulators and clinical applications. Biochem Pharmacol. 2001. 61:253–270.
Article14. Lu J, Kim SH, Jiang C, Lee H, Guo J. Oriental herbs as a source of novel anti-androgen and prostate cancer chemopreventive agents. Acta Pharmacol Sin. 2007. 28:1365–1372.
Article15. Lv N, Koo JH, Yoon HY, Yu J, Kim KA, Choi IW, Kwon KB, Kwon KS, Kim HU, Park JW, Park BH. Effect of Angelica gigas extract on melanogenesis in B16 melanoma cells. Int J Mol Med. 2007. 20:763–767.
Article16. Ma Y, Han H, Eun JS, Kim HC, Hong JT, Oh KW. Sanjoinine A isolated from Zizyphi Spinosi Semen augments pentobarbital-induced sleeping behaviors through the modification of GABA-ergic systems. Biol Pharm Bull. 2007. 30:1748–1753.
Article17. Meng H, Guo J, Sun JY, Pei JM, Wang YM, Zhu MZ, Huang C. Angiogenic effects of the extracts from Chinese herbs: Angelica and Chuanxiong. Am J Chin Med. 2008. 36:541–554.
Article18. Park JH, Lee HJ, Koh SB, Ban JY, Seong YH. Protection of NMDA-induced neuronal cell damage by methanol extract of Zizyphi spinosi semen in cultured rat cerebellar granule cells. J Ethnopharmacol. 2004. 95:39–45.
Article19. Singh RP, Agarwal R. Mechanisms of action of novel agents for prostate cancer chemoprevention. Endocr Relat Cancer. 2006. 13:751–778.
Article20. Stamenkovic I. Extracellular matrix remodeling: The role of matrix metalloproteinases. J Pathol. 2003. 200:448–464.21. Stetler-Stevenson WG. Matrix metalloproteinases in angiogenesis: A moving target for therapeutic intervention. J Clin Invest. 1999. 103:1237–1241.
Article22. Wang LE, Bai YJ, Shi XR, Cui XY, Cui SY, Zhang F, Zhang QY, Zhao YY, Zhang YH. Spinosin, a C-glycoside flavonoid from semen Zizhiphi Spinozae, potentiated pentobarbital-induced sleep via the serotonergic system. Pharmacol Biochem Behav. 2008. 90:399–403.
Article23. Weinstat-Saslow D, Steeg PS. Angiogenesis and colonization in the tumor metastatic process: basic and applied advances. FASEB J. 1994. 8:401–407.
Article24. Yun YJ, Lee B, Hahm DH, Kang SK, Han SM, Lee HJ, Pyun KH, Shim I. Neuroprotective effect of palmul-chongmyeongtang on ischemia-induced learning and memory deficits in the rat. Biol Pharm Bull. 2007. 30:337–342.
Article25. Zhang M, Ning G, Shou C, Lu Y, Hong D, Zheng X. Inhibitory effect of jujuboside A on glutamate-mediated excitatory signal pathway in hippocampus. Planta Med. 2003. 69:692–695.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Studies on the Chemical Constituents from the Seeds of Zizyphus jujuba var. inermis
- Influence of angelicae gigantis radix on the immune system(II): stimulation of hemolytic plaque forming cells by in vivo treatment -
- Ethanolic Extract of the Seed of Zizyphus jujuba var. spinosa Ameliorates Cognitive Impairment Induced by Cholinergic Blockade in Mice
- Isolation of Emericella Nidulans var. Latus (Imperfect State, Aspergillus Nidulans var. Latus) from a Patient with Healed Pulmonary Tuberculosis
- Anti-Influenza Activity of Betulinic Acid from Zizyphus jujuba on Influenza A/PR/8 Virus