Nutr Res Pract.
2007 Dec;1(4):254-259.
Childbearing women of twenty and under are at greater risk than those of twenty-five and over for compromised folate status
- Affiliations
-
- 1Department of Food and Nutrition, Chonnam National University, Gwangju, 500-757 Korea. limhs@chonnam.ac.kr
Abstract
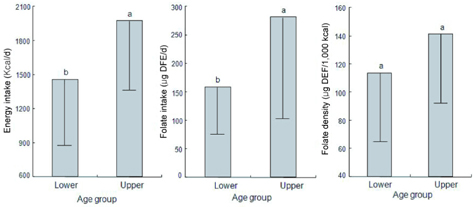
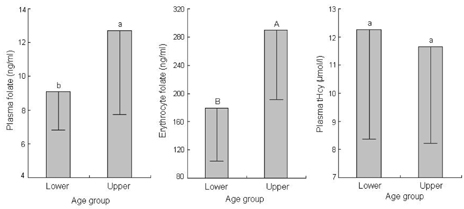
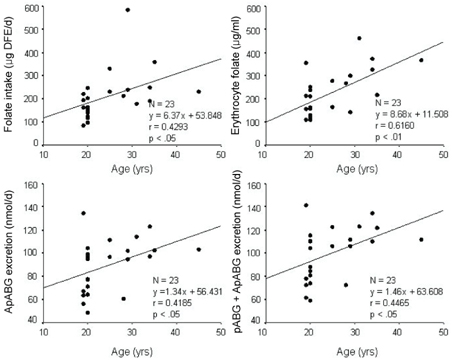
- This study assessed folate intakes, folate concentrations in plasma and erythrocytes, plasma total homocysteine (tHcy) concentration, and urinary excretion of folate metabolites in Korean women with childbearing potential. A total of 23 women voluntarily participated in this study. Precise dietary intakes for 3 consecutive days were determined by weighing all foods consumed and folate intake was calculated using a computer-aided dietary analysis system. Folate concentration of plasma and erythrocytes was determined by a microbiological method. Plasma tHcy concentration was assayed using an HPLC analysis method. Urine excreted over the same period of time was collected and folate catabolites, para-aminobenzoylglutamate (pABG) and para-acetamidobenzoylglutamate (ApABG), were evaluated using a reverse-phase HPLC method after affinity chromatography. Young women of 20 and under were likely to consume less folate with low energy intake, had lower folate concentration in plasma and erythrocytes, and excreted a lesser amount of ApABG and total folate catabolites than women of 25 years and over. The results of this study confirmed that young Korean women with childbearing potential, especially those under 21 years of age, might be at risk for compromised folate status due to insufficient folate intakes from inadequate energy consumption.
MeSH Terms
Figure
Reference
-
1. Ahn HS, Jeong EY, Kim SY. Studies on plasma homocysteine concentration and nutritional status of vitamin B6, B12 and folate in college women. Korean Journal of Nutrition. 2002. 35:37–44.2. Araki A, Sako Y. Determination of free and total homocysteine in human plasma by HPLC with fluorescence detection. J Chromatogr. 1987. 422:43–52.
Article3. Bakker RC, Brandjes DPM. Hyperhomocysteinemia and associated disease. Pharm World Sci. 1997. 19:126–132.4. Bonin MM, Bretzlaff JA, Therrien SA, Rowe BH. Knowledge of periconceptional folic acid for the prevention of neural tube defects; the missing links. Arch Fam Med. 1998. 7:438–442.
Article5. Brouwer IA, van Dusseldorp M, Thomas CMG, Duran M, Hautvast JGAJ, Eskes TKAB, Steegers-Theunissen RPM. Low-dose folic acid supplementation decreases plasma homocysteine concentrations: a randomized trial. Am J Clin Nutr. 1999. 69:99–104.
Article6. Caudill MA, Gregory JF III, Huston AD, Bailey LB. Folate catabolism in pregnant and nonpregnant women with controlled folate intakes. J Nutr. 1998. 128:204–208.
Article7. Chinebuah B, Perez-Escamilla RP. Unplanned pregnancies are associated with less likelihood of prolonged breast-feeding among primiparous women in Ghana. J Nutr. 2001. 131:1247–1249.
Article8. Folic acid and the prevention of neural tube defects. Department of Health Report from an Expert Advisory Group. 1992. London. UK: Department of Health.9. Estrada-Rodgers L, Levy GN, Weber WW. Substrate selectivity of mouse N-acetyltransferase 1, 2, and 3 expressed in COS-1 cells. Drug Metab Dispos. 1998. 26:502–505.10. Geoghegan FL, McPartlin JM, Weir DG, Scott JM. Paraacetamidobenzoylglutamate is a suitable indicator of folate catabolism in rats. J Nutr. 1995. 125:2563–2570.11. Gregory JF, Swendseid ME, Jacob RA. Urinary excretion of folate catabolites responds to changes in folate intake more slowly than plasma folate and homocysteine concentrations and lymphocyte DNA methylation in postmenopausal women. J Nutr. 2000. 130:2949–2952.
Article12. Grimes DA. Unplanned pregnancies in the US. Obstet Gynecol. 1986. 67:438–442.13. Herbert V. Making sense of laboratory tests of folate status: folate requirements to sustain normality. Am J Hematol. 1987. 26:199–207.
Article14. Herbert V. Picciano MF, Stokstad ELR, Gregory JP, editors. Development of human folate deficiency. Evaluation of Folic Acid Metabolism in Nutrition and Disease. 1990. New York. USA: Alan R. Liss;195–210.15. Hyun TS, Han YH. Comparison of folate intake and food sources in college students using the 6th vs. 7th nutrient database. Korean Journal of Nutrition. 2001. 34:797–808.16. Hyun TS, Han YH, Lim EY. Blood folate level determined by a microplate reader and folate intake measured by a weighed food record. Korean Journal of Community Nutrition. 1999. 4:512–520.17. Jacob RA, Wu MM, Henning SM, Swenseid ME. Homocysteine increases as folate decreases in plasma of healthy men during short-term dietary folate and methyl group restriction. J Nutr. 1994. 124:1072–1080.
Article18. Kim Y, Kim K, Chang N. Dietary folate intake of Korean women of childbearing age. Korean Journal of Nutrition. 1999. 32:585–591.19. Kownacki-Brown PA, Wang C, Bailey LB, Toth JP, Gregory JF III. Urinary excretion of deuterium-labeled folate and the metabolite p-aminobenzoylglutamate in humans. J Nutr. 1993. 123:1101–1108.20. Lim HS, Jin HO, Lee JA. Dietary intakes and status of folate in Korean women of childbearing potential. Korean Journal of Nutrition. 2000. 33:296–303.21. Lim HS, Kang SA, Lee JI, Jin HO. Low intakes of energy, folate, iron, and calcium of childbearing Korean women. Ecol Food Nutr. 2002. 41:401–403.
Article22. McNulty H, Cuskelly GS, Ward M. Response of red blood cell folate to intervention; implications for folate recommendations for the prevention of neural tube defects. Am J Clin Nutr. 2000. 71:1308S–1311S.
Article23. McPartlin J, Halligan A, Scott JM, Darling M, Weir DG. Accelerated folate breakdown in pregnancy. Lancet. 1993. 341:148–149.
Article24. McPartlin JM, Courtney G, McNulty H, Weir DG, Scott JM. The quantitative analysis of endogenous folate catabolites in human urine. Anal Biochem. 1992. 206:256–261.
Article25. Minchin RF. Acetylation of p-aminobenzoylglutamate, a folic acid catabolite, by recombinant human arylamine N-acetyltransferase and U937 cells. Biochem J. 1995. 307:1–3.
Article26. Moyers S, Bailey LB. Fetal malformations and folate metabolism; review of recent evidence. Nutr Rev. 2001. 59:215–235.
Article27. O'Keefe CA, Bailey LB, Thomas EA, Hofler SA, Davis BA, Cerda JJ, Gregory JF. Controlled dietary folate affects folate status in nonpregnant women. J Nutr. 1995. 125:2717–2725.28. Sauberlich HE, Kretsch MJ, Skala JH, Johnson HL, Taylor PC. Folate requirement and metabolism in nonpregnant women. Am J Clin Nutr. 1987. 46:1016–1028.
Article29. Scholl TO, Johnson WG. Folic acid; influence on the outcome of pregnancy. Am J Clin Nutr. 2000. 71:1295S–1303S.
Article30. Selhub J, Ahmad O, Rosenberg IH. Preparation and use of affinity columns with bovine milk-folate binding protein covalently linked to Sepaharose 4B. Methods Enzymol. 1980. 66:686–690.31. Tamura T. Picciano MF, Stokstad ELR, Gregory JF, editors. Microbiological assay of folates. Folic Acid Metabolism in Health and Disease. 1990. New York. USA: John Wiley & Sons;121–137.32. Tu A, Chanarin I, Slavin G, Levi AJ. Folate deficiency in the alcoholic; its relationship to clinical and hematological abnormalities, liver disease, and folate stores. Br J Haematol. 1975. 29:269–278.33. Vollset SE, Refsum H, Irgens LM, Emblem BM, Tverdal A, Gjessing HK, Monsen ALB, Ueland PM. Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes; the Hordaland Homocysteine Study. Am J Clin Nutr. 2000. 71:962–968.
Article34. Wolfe JM, Bailey LB, Garcia KH, Therkabue DW, Gregory JF III, Kauwell GP. Folate catabolite excretion is responsive to changes in dietary folate intake in elderly women. Am J Clin Nutr. 2003. 77:919–923.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Folate Content of Human Milk During Extended Lactation and Folate Nutritional Status of Lactating Women in Korea
- Vitamin B Status and Serum Homocysteine Levels in Infertile Women
- A Longitudinal Study on Maternal Iron and Folate Status During and After Pregnancy in Korean Women
- Folate food source, usual intake, and folate status in Korean adults
- Awareness, knowledge, and use of folic acid among non-pregnant Korean women of childbearing age