Lab Med Online.
2011 Jan;1(1):57-63.
Evaluation of ABO Antibody Titration Using Tube and Column Agglutination Techniques
- Affiliations
-
- 1Department of Laboratory Medicine, School of Medicine, Korea University, Seoul, Korea. malarim@korea.ac.kr
Abstract
- BACKGROUND
ABO antibody titration is useful for the evaluation of ABO-incompatible bone marrow or solid organ transplantations, yet the results quite vary between different test methods used. We compared the results of microcolumn agglutination and tube methods.
METHODS
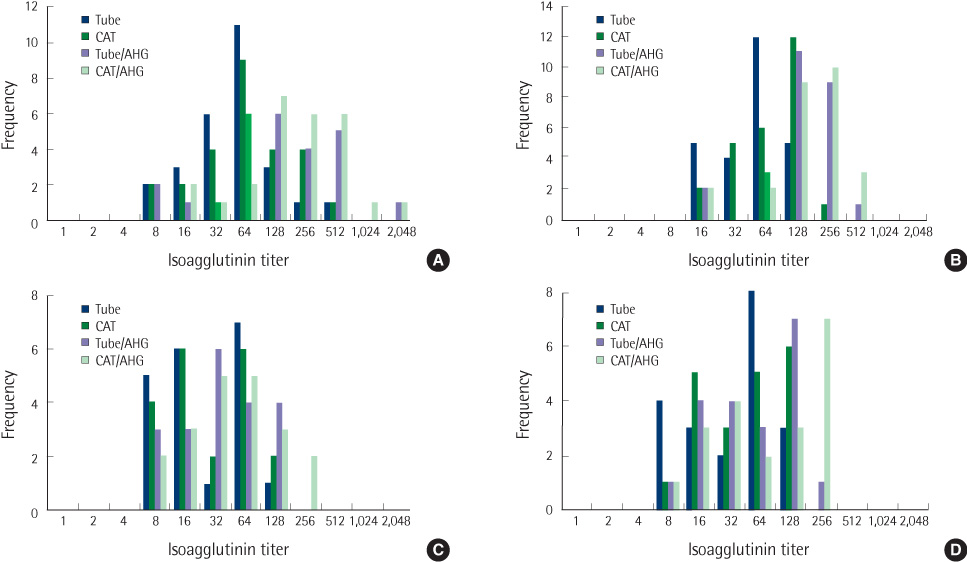
Anti-A and anti-B isoagglutionin titers were determined in 63 healthy individuals (23 O, 20 A, and 20 B blood groups) using 4 different methods: immediate spin tube (tube), microcolumn agglutination without anti-human globulin (AHG) (CAT), tube with AHG (tube-AHG) and microcolumn agglutination with AHG (CAT-AHG).
RESULTS
The median (range) titers of anti-A and anti-B in group O individuals by tube, CAT, tube-AHG, and CAT-AHG methods were 64 (8-512), 64 (8-512), 128 (8-2,048), and 128 (16-2,048); 64 (16-128), 128 (16-256), 128 (16-512), and 256 (16-512), respectively. The median (range) titers of anti-A in group B and anti-B in group A individuals by the four methods were 64 (16-128), 128 (8-128), 128 (8-256), and 256 (8-256); 64 (8-128), 64 (8-128), 32 (8-128), and 64 (8-256), respectively. The isoagglutinin titer measured by CAT-AHGmethod was the highest. The titers measured by CAT and CAT-AHG methods were 0-1 titer higher than those by tube and tube-AHG methods, respectively. Whatever method was used, the isoagglutinin titers were higher in women than in men.
CONCLUSIONS
CAT-AHG was the most sensitive method among the four methods tested. Since AHG titer values are critical for the clinical management and CAT has less manual procedures than tube method, CAT-AHG method could be used for the standardization of ABO antibody titration in different institutions.
Keyword
Figure
Reference
-
1. Beadling WV, Cooling L. McPherson RA, Pincus MR, editors. Immunohematology. Clinical diagnosis and management by laboratory methods. 2007. 21st ed. Philadelphia: Saunders Elseviver;623–660.2. Han KS, Park MH, editors. Transfusion medicine. 2006. 3rd ed. Seoul: Korea Medical Publisher;165.3. Song TJ, Lim CS, Park IS, Lee KN, Jung KH, Choi SY, et al. ABO blood type incompatible liver transplantation in a child. J Korean Surg Soc. 1998. 55:604–608.4. Shimmura H, Tanabe K, Ishikawa N, Tokumoto T, Takahashi K, Toma H. Role of anti-A/B antibody titers in results of ABO-incompatible kidney transplantation. Transplantation. 2000. 70:1331–1335.
Article5. Lee SH, Kwon SW, Lee JH, Lee KH, Kim WK, Kim SH, et al. Isoagglutinin titer in major ABO incompatible bone marrow transplantation. Korean J Blood Transfus. 1997. 8:167–176.6. Kobayashi T, Saito K. A series of surveys on assay for anti-A/B antibody by Japanese ABO-incompatible Transplantation Committee. Xenotransplantation. 2006. 13:136–140.
Article7. Kumlien G, Wilpert J, Safwenberg J, Tyden G. Comparing the tube and gel techniques for ABO antibody titration, as performed in three European centers. Transplantation. 2007. 84:S. S17–S19.
Article8. Kang MG, Lee SJ, Oh JS, Lim YA. Comparison of ABO isoagglutinin titers by different tube hemagglutination techniques. Korean J Blood Transfus. 2009. 20:227–234.9. Walker PS. Roback JD, Combs MR, editors. Identification of antibodies to red cell antigens. Technical manual. 2008. 16th ed. Bethesda: American Association of Blood Banks;483–484.10. Kim JS, Ryu KH, Lee HK, Kim DW. Evaluation of ABO isoagglutinin titers in Korean adults. Korean J Blood Transfus. 1997. 8:119–124.11. Shirey RS, Cai W, Montgomery RA, Chhibber V, Ness PM, King KE. Streamlining ABO antibody titrations for monitoring ABO-incompatible kidney transplants. Transfusion. 2010. 50:631–634.
Article12. Tobian AA, Shirey RS, Montgomery RA, Ness PM, King KE. The critical role of plasmapheresis in ABO-incompatible renal transplantation. Transfusion. 2008. 48:2453–2460.
Article13. AuBuchon JP, de Wildt-Eggen J, Dumont LJ. Reducing the variation in performance of antibody titrations. Vox Sang. 2008. 95:57–65.
Article14. Rosner ER, Pirofsky B, Sheth K. The influence of dialysis in 2-mercaptoethanol reduction of erythrocyte antibodies. Transfusion. 1974. 14:47–50.
Article15. King KE, Warren DS, Samaniego-Picota M, Campbell-Lee S, Montgomery RA, Baldwin WM 3rd. Antibody, complement and accommodation in ABO-incompatible transplants. Curr Opin Immunol. 2004. 16:545–549.
Article16. Mollison PL, Engelfriet CP, Contreras M. Mollison PL, Engelfriet CP, editors. ABO, Lewis, Ii and P groups. Blood transfusion in clinical medicine. 1993. 9th ed. Oxford: Blackwell Scientific Publications;148–203.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Column Agglutination Technique and Tube Test for ABO Antibody Titration and Crossmatching
- Usefulness of column agglutination test for irregular antibody screening and identification
- Comparison of Total and IgG ABO Antibody Titers in Healthy Individuals by Using Tube and Column Agglutination Techniques
- Evaluation of the Automated Immunohematology Analyzer ORTHO VISION for ABO Antibody Titration
- Evaluation of the Automated QWALYS-3 System for ABO and RhD Grouping and Unexpected Antibody Screening