Lab Anim Res.
2014 Sep;30(3):136-141. 10.5625/lar.2014.30.3.136.
Experimental model of tympanic colic (acute abdomen) in chinchillas (Chinchilla lanigera)
- Affiliations
-
- 1Centro Ciencias da Saude, Universidade de Cruz Alta, Cruz Alta, Brazil.
- 2Laboratorio de Neurobiologia Comparada, Departamento de Fisiologia, Instituto de Ciencias Basicas da Saude, Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil. zancan@ufrgs.br
- 3Programa de Pos-Graduacao em Neurociencias, Instituto de Ciencias Basicas da Saude (ICBS), Universidade Federal do Rio Grande do Sul, Porto Alegre, Brazil.
- KMID: 2312125
- DOI: http://doi.org/10.5625/lar.2014.30.3.136
Abstract
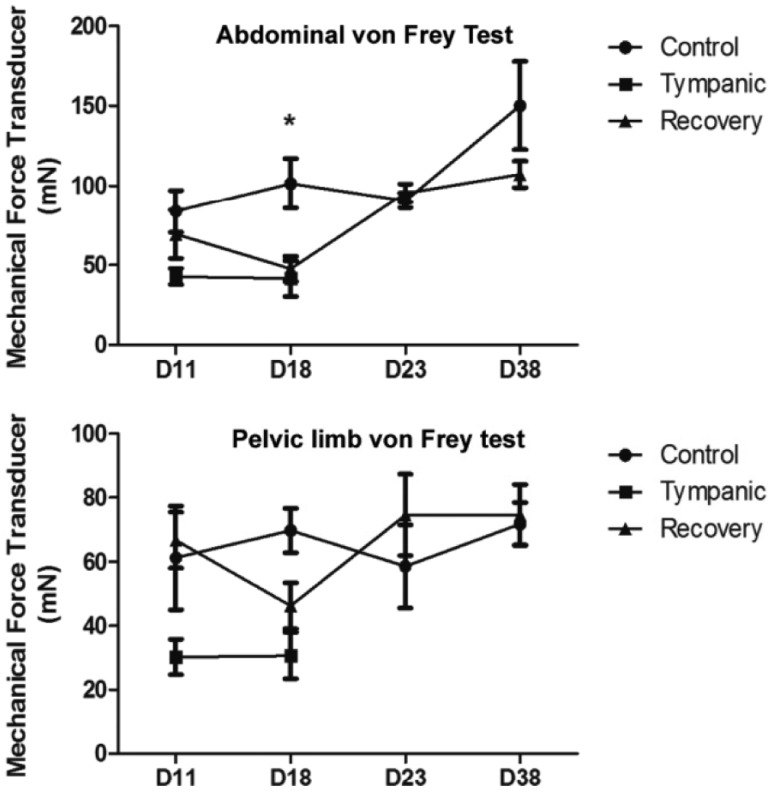
- Digestive disorders caused by sudden changes in diet or inappropriate diet are among the most common disorders of the digestive system. Cecal or intestinal tympany, one consequence of inappropriate diet, is characterized by the accumulation of gases, marked distension of the cecum and colon and the induction of inflammatory processes. To know the effects of intestinal tympany on the enteric plexuses, we developed a method of experimental tympanic colic (TC) in the Chinchilla lanigera. This species was used in view of its susceptibility to TC. TC was induced with a diet rich in alfalfa associated with grain overload for two weeks. Physical and clinical examination including the von Frey test confirmed the diagnosis. The chinchillas with acute abdomen were treated with 1% ketoprofen and resumption of a balanced diet. Necropsy and histopathological analysis showed tympany-induced alterations mainly in the cecum and colon. After treatment, the control conditions were restored. The TC protocol is proposed as an experimental approach designed to aid the study of the effects of acute intestinal inflammation and obstruction caused by an inappropriate diet.
MeSH Terms
Figure
Reference
-
1. Tinker MK, White NA, Lessard P, Thatcher CD, Pelzer KD, Davis B, Carmel DK. Prospective study of equine colic incidence and mortality. Equine Vet J. 1997; 29(6):448–453. PMID: 9413717.
Article2. Hoefer HL. Chinchillas. Vet Clin North Am Small Anim Pract. 1994; 24(1):103–111. PMID: 8109069.
Article3. Donnelly TM, Schaeffer DO. Disease problems of guinea pigs and chinchillas. In : Hillyer EV, Quesenberry KQ, editors. Ferrets, Rabbits, and Rodents, Clinical Medicine and Surgery. 1997. p. 270–281.4. Gonçalves S, Julliand V, Leblond A. Risk factors associated with colic in horses. Vet Res. 2002; 33(6):641–652. PMID: 12498565.5. White NA II. Causes and risks for colic. AAEP Proceedings. 2006; 52:115–119.6. Kunze WA, Furness JB, Bertrand PP, Bornstein JC. Intracellular recording from myenteric neurons of the guinea-pig ileum that respond to stretch. J Physiol. 1998; 506:827–842. PMID: 9503341.
Article7. Saper CB. The central autonomic nervous system: conscious visceral perception and autonomic pattern generation. Annu Rev Neurosci. 2002; 25:433–469. PMID: 12052916.
Article8. Jänig W. The integrative action of the autonomic nervous system: Neurobiology of homeostasis. Cambridge: Cambridge University Press;2006. p. 168–208.9. Castro TF, Dummer RJ, Rickes EM, Pereira MAM. Morphological, morphometric and topographical description of the digestive tract in Chinchilla lanigera. Braz J Vet Res Anim Sci. 2010; 47:86–94.10. Martinez-Pereira MA, Rickes EM. The spinal nerves that constitute the lumbosacral plexus and their distribution in the chinchilla. J S Afr Vet Assoc. 2011; 82(3):150–154. PMID: 22332298.
Article11. Brown TA, Harrison RV. Responses of neurons in chinchilla auditory cortex to frequency-modulated tones. J Neurophysiol. 2009; 101(4):2017–2029. PMID: 19211659.
Article12. Jakubów K, Gromadzka-Ostrowska J, Zalewska B. Seasonal changes in the haematological indices in peripheral blood of chinchilla (Chinchilla laniger L.). Comp Biochem Physiol A Comp Physiol. 1984; 78(4):845–853. PMID: 6149060.
Article13. Busso JM, Ponzio MF, Fiol de Cuneo M, Ruiz RD. Reproduction in chinchilla (Chinchilla lanigera): current status of environmental control of gonadal activity and advances in reproductive techniques. Theriogenology. 2012; 78(1):1–11. PMID: 22541170.
Article14. O'Malley B. Introduction to small mammals. Clinical anatomy and physiology of exotic species. New York: Saunders;2005. p. 165–171.15. Klaphake E. Common rodent procedures. Vet Clin North Am Exot Anim Pract. 2006; 9(2):389–413. PMID: 16759953.
Article16. Weiss DJ, Evanson OA, McClenahan D, Fagliari JJ, Dunnwiddie CT, Wells RE. Effect of a competitive inhibitor of platelet aggregation on experimentally induced laminitis in ponies. Am J Vet Res. 1998; 59(7):814–817. PMID: 9659543.17. Wolf P, Schröder A, Wenger A, Kamphues J. The nutrition of the chinchilla as a companion animal--basic data, influences and dependences. J Anim Physiol Anim Nutr (Berl). 2003; 87(3-4):129–133. PMID: 14511138.18. Clarke RT, Reid CS. Foamy bloat of cattle. A review. J Dairy Sci. 1974; 57(7):753–785. PMID: 4601637.
Article19. Vieira MEQ, Costa C, Silveira AC, Arrigoni MB. Porcentagens de saponinas e taninos em vinte e oito cultivares de alfafa (Medicago sativa L.) em duas épocas de corte -Botucatu- SP. Rev Bras Zootec. 2001; 30:1432–1438.20. Johnson-Delaney CA. Exotic Companion Medicine Handbook for Veterinarians. Florida: Zoological Education Network;2008. p. 98.21. Sanovic S, Lamb DP, Blennerhassett MG. Damage to the enteric nervous system in experimental colitis. Am J Pathol. 1999; 155(4):1051–1057. PMID: 10514387.
Article22. Sharkey KA, Kroese AB. Consequences of intestinal inflammation on the enteric nervous system: neuronal activation induced by inflammatory mediators. Anat Rec. 2001; 262(1):79–90. PMID: 11146431.
Article23. Linden DR, Sharkey KA, Ho W, Mawe GM. Cyclooxygenase-2 contributes to dysmotility and enhanced excitability of myenteric AH neurones in the inflamed guinea pig distal colon. J Physiol. 2004; 557:191–205. PMID: 15020692.
Article24. Eckert BS, Funkler GR, Rodrigues SV. Case report: Acute abdomen in chinchilla. UFRGS;2011.25. Collins SM. The immunomodulation of enteric neuromuscular function: implications for motility and inflammatory disorders. Gastroenterology. 1996; 111(6):1683–1699. PMID: 8942751.
Article26. Lomax AE, Fernández E, Sharkey KA. Plasticity of the enteric nervous system during intestinal inflammation. Neurogastroenterol Motil. 2005; 17(1):4–15. PMID: 15670258.
Article27. Vasina V, Barbara G, Talamonti L, Stanghellini V, Corinaldesi R, Tonini M, De Ponti F, De Giorgio R. Enteric neuroplasticity evoked by inflammation. Auton Neurosci. 2006; 126-127:264–272. PMID: 16624634.
Article28. Mawe GM, Strong DS, Sharkey KA. Plasticity of enteric nerve functions in the inflamed and postinflamed gut. Neurogastroenterol Motil. 2009; 21(5):481–491. PMID: 19368664.
Article29. Valentine JF, Tannahill CL, Stevenot SA, Sallustio JE, Nick HS, Eaker EY. Colitis and interleukin 1beta up-regulate inducible nitric oxide synthase and superoxide dismutase in rat myenteric neurons. Gastroenterology. 1996; 111(1):56–64. PMID: 8698225.
Article30. Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989; 96(3):795–803. PMID: 2914642.
Article31. Reinshagen M, Rohm H, Steinkamp M, Lieb K, Geerling I, Von Herbay A, Flämig G, Eysselein VE, Adler G. Protective role of neurotrophins in experimental inflammation of the rat gut. Gastroenterology. 2000; 119(2):368–376. PMID: 10930372.
Article32. Barbara G, Vallance BA, Collins SM. Persistent intestinal neuromuscular dysfunction after acute nematode infection in mice. Gastroenterology. 1997; 113(4):1224–1232. PMID: 9322517.
Article33. Chen Z, Suntres Z, Palmer J, Guzman J, Javed A, Xue J, Yu JG, Cooke H, Awad H, Hassanain HH, Cardounel AJ, Christofi FL. Cyclic AMP signaling contributes to neural plasticity and hyperexcitability in AH sensory neurons following intestinal Trichinella spiralis-induced inflammation. Int J Parasitol. 2007; 37(7):743–761. PMID: 17307183.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histologic Changes of Lateral Semicircular Canal after Transection and Occlusion with Various Materials in Chinchillas
- Treatment for Acute Tympanic Membrane Perforation
- Retrograde labeling of efferent vestibular neurons in the chinchilla
- Central projections of the vestibular nerve in chinchilla: I. scratch method an extracellular labelling technique
- Colo-Colic Intussusception as the Presenting Feature of Neutropenic Typhlitis during Induction Phase of Acute Lymphoblastic Leukemia in an 8-Year-Old Child