J Breast Cancer.
2016 Jun;19(2):191-198. 10.4048/jbc.2016.19.2.191.
Breast Magnetic Resonance Imaging for Assessment of Internal Mammary Lymph Node Status in Breast Cancer
- Affiliations
-
- 1Division of Radiology, Seoul St. Mary's Hospital, The Catholic University of Korea College of Medicine, Seoul, Korea. rad-ksh@catholic.ac.kr
- KMID: 2308971
- DOI: http://doi.org/10.4048/jbc.2016.19.2.191
Abstract
- PURPOSE
The purpose of this study was to assess magnetic resonance imaging (MRI) features of malignant internal mammary lymph nodes (IMLNs) and benign IMLNs in breast cancer patients.
METHODS
From 2009 to 2014, the records of 85 patients with IMLNs were archived using MRI report data; 26 patients with small size (long axis diameter <5 mm) nodes were subsequently excluded. The current study evaluated internal mammary lymph nodes in 59 patients who underwent breast MRI for breast cancer staging and for posttherapy follow-up. All MRI findings were retrospectively evaluated. Malignancy was determined based on pathologic examination and positron emission tomography computed tomography findings. Independent t-tests, Mann-Whitney U tests, chi-square tests, and receiver operating characteristics (ROC) curve analysis were used.
RESULTS
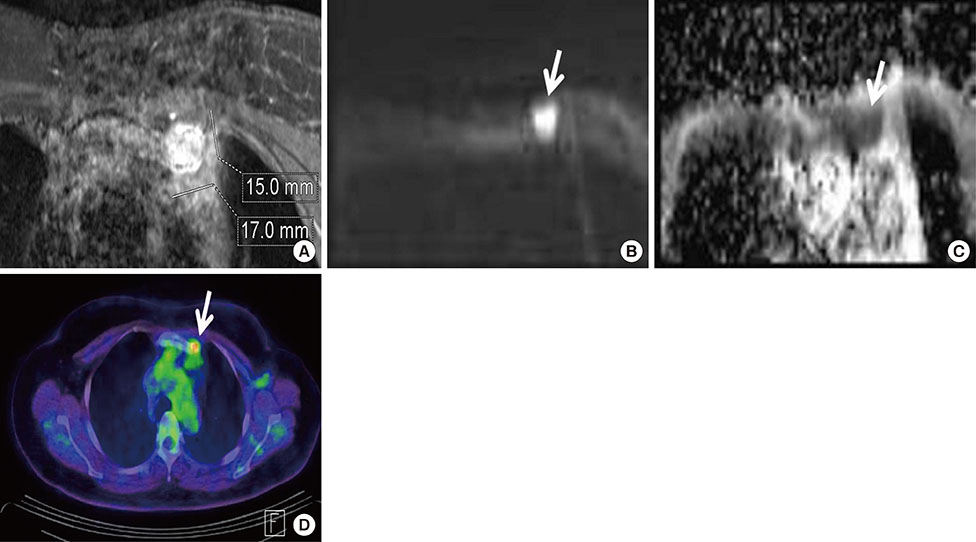
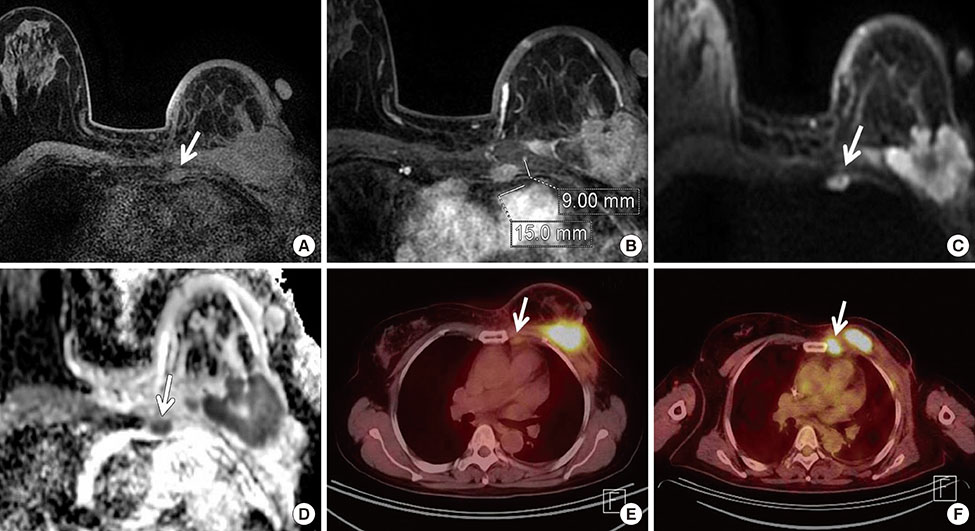
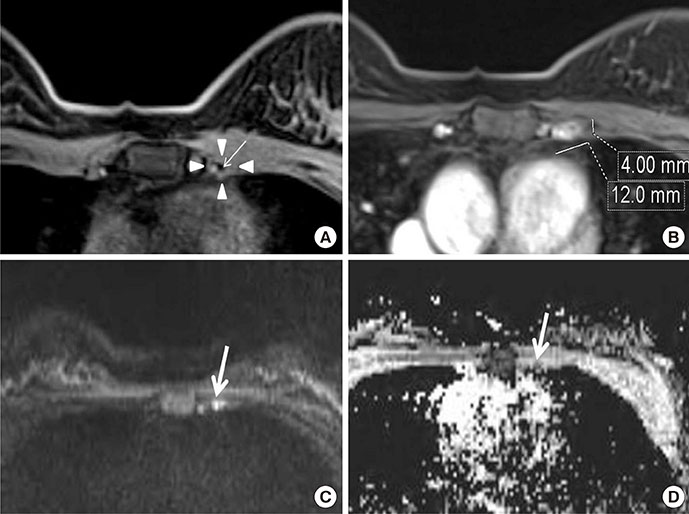
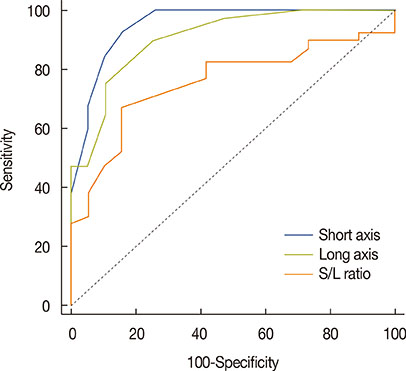
Among MRI features, there were statistically significant differences between benign and malignant IMLN groups, in short axis length (3.6±1.3 vs. 8.2±2.9 mm, respectively), long axis length (8.1±2.4 vs. 14.5±4.8 mm, respectively), short/long axis ratio (0.45±0.10 vs. 0.59±0.17, respectively), absent fatty hilum (mean, 0% vs. 95%, respectively), and restricted diffusion (15.8% vs. 85.0%, respectively) (p<0.050). Multiplicity and location of intercostal spaces was not different between the two groups. Short axis length was the most discriminative variable for predicting metastatic nodes (area under the ROC curve, 0.951; threshold, 4 mm; sensitivity, 92.5%; specificity, 84.2%).
CONCLUSION
Conventional MRI and diffusion-weighted MRI are helpful to detect metastasis of internal mammary lymph nodes in breast cancer.
MeSH Terms
Figure
Reference
-
1. Li Z, Gu X, Tong J, Liu B, Sun L, Gao X, et al. A meta-analysis of internal mammary lymph node metastasis in breast cancer patients. Onkologie. 2013; 36:747–752.
Article2. Cody HS 3rd. Clinical significance and management of extra-axillary sentinel lymph nodes: worthwhile or irrelevant? Surg Oncol Clin N Am. 2010; 19:507–517.
Article3. Veronesi U, Marubini E, Mariani L, Valagussa P, Zucali R. The dissection of internal mammary nodes does not improve the survival of breast cancer patients: 30-year results of a randomised trial. Eur J Cancer. 1999; 35:1320–1325.
Article4. Cranenbroek S, van der Sangen MJ, Kuijt GP, Voogd AC. Diagnosis, treatment and prognosis of internal mammary lymph node recurrence in breast cancer patients. Breast Cancer Res Treat. 2005; 89:271–275.5. Maalej M, Gargouri W, Kochbati L, Nasr C, Frikha H, Hentati D, et al. Internal mammary lymph node invasion in breast cancer: myth or reality? Tunis Med. 2009; 87:319–322.6. Noguchi M. Internal mammary sentinel node biopsy for breast cancer: is it practicable and relevant? (Review). Oncol Rep. 2002; 9:461–468.
Article7. An YY, Kim SH, Kang BJ, Lee AW. Comparisons of positron emission tomography/computed tomography and ultrasound imaging for detection of internal mammary lymph node metastases in patients with breast cancer and pathologic correlation by ultrasound-guided biopsy procedures. J Ultrasound Med. 2015; 34:1385–1394.
Article8. Chen RC, Lin NU, Golshan M, Harris JR, Bellon JR. Internal mammary nodes in breast cancer: diagnosis and implications for patient management: a systematic review. J Clin Oncol. 2008; 26:4981–4989.
Article9. Kinoshita T, Odagiri K, Andoh K, Doiuchi T, Sugimura K, Shiotani S, et al. Evaluation of small internal mammary lymph node metastases in breast cancer by MRI. Radiat Med. 1999; 17:189–193.10. Seo MJ, Lee JJ, Kim HO, Chae SY, Park SH, Ryu JS, et al. Detection of internal mammary lymph node metastasis with (18)F-fluorodeoxyglucose positron emission tomography/computed tomography in patients with stage III breast cancer. Eur J Nucl Med Mol Imaging. 2014; 41:438–445.
Article11. Kim EJ, Kim SH, Kang BJ, Choi BG, Song BJ, Choi JJ. Diagnostic value of breast MRI for predicting metastatic axillary lymph nodes in breast cancer patients: diffusion-weighted MRI and conventional MRI. Magn Reson Imaging. 2014; 32:1230–1236.
Article12. Mack M, Chetlen A, Liao J. Incidental internal mammary lymph nodes visualized on screening breast MRI. AJR Am J Roentgenol. 2015; 205:209–214.
Article13. Ray KM, Munir R, Wisner DJ, Azziz A, Holland BC, Kornak J, et al. Internal mammary lymph nodes as incidental findings at screening breast MRI. Clin Imaging. 2015; 39:791–793.
Article14. He N, Xie C, Wei W, Pan C, Wang W, Lv N, et al. A new, preoperative, MRI-based scoring system for diagnosing malignant axillary lymph nodes in women evaluated for breast cancer. Eur J Radiol. 2012; 81:2602–2612.
Article15. Yoshimura G, Sakurai T, Oura S, Suzuma T, Tamaki T, Umemura T, et al. Evaluation of axillary lymph node status in breast cancer with MRI. Breast Cancer. 1999; 6:249–258.
Article16. Luciani A, Dao TH, Lapeyre M, Schwarzinger M, Debaecque C, Lantieri L, et al. Simultaneous bilateral breast and high-resolution axillary MRI of patients with breast cancer: preliminary results. AJR Am J Roentgenol. 2004; 182:1059–1067.
Article17. Mortellaro VE, Marshall J, Singer L, Hochwald SN, Chang M, Copeland EM, et al. Magnetic resonance imaging for axillary staging in patients with breast cancer. J Magn Reson Imaging. 2009; 30:309–312.
Article18. Fornasa F, Nesoti MV, Bovo C, Bonavina MG. Diffusion-weighted magnetic resonance imaging in the characterization of axillary lymph nodes in patients with breast cancer. J Magn Reson Imaging. 2012; 36:858–864.
Article19. Junping W, Tongguo S, Yunting Z, Chunshui Y, Renju B. Discrimination of axillary metastatic from nonmetastatic lymph nodes with PROPELLER diffusion-weighted MR imaging in a metastatic breast cancer model and its correlation with cellularity. J Magn Reson Imaging. 2012; 36:624–631.
Article20. Wang J, Liao Q, Zhang Y, Yu C, Bai R, Sun H. Differential diagnosis of axillary inflammatory and metastatic lymph nodes in rabbit models by using diffusion-weighted imaging: compared with conventional magnetic resonance imaging. Korean J Radiol. 2012; 13:458–466.
Article21. Eubank WB, Mankoff DA, Takasugi J, Vesselle H, Eary JF, Shanley TJ, et al. 18Fluorodeoxyglucose positron emission tomography to detect mediastinal or internal mammary metastases in breast cancer. J Clin Oncol. 2001; 19:3516–3523.
Article22. Segaert I, Mottaghy F, Ceyssens S, De Wever W, Stroobants S, Van Ongeval C, et al. Additional value of PET-CT in staging of clinical stage IIB and III breast cancer. Breast J. 2010; 16:617–624.
Article23. Hindié E, Groheux D, Hennequin C, Zanotti-Fregonara P, Vercellino L, Berenger N, et al. Lymphoscintigraphy can select breast cancer patients for internal mammary chain radiotherapy. Int J Radiat Oncol Biol Phys. 2012; 83:1081–1088.
Article24. van der Ent FW, Kengen RA, van der Pol HA, Povel JA, Stroeken HJ, Hoofwijk AG. Halsted revisited: internal mammary sentinel lymph node biopsy in breast cancer. Ann Surg. 2001; 234:79–84.
Article25. Donegan WL. The influence of untreated internal mammary metastases upon the course of mammary cancer. Cancer. 1977; 39:533–538.
Article26. Huang O, Wang L, Shen K, Lin H, Hu Z, Liu G, et al. Breast cancer subpopulation with high risk of internal mammary lymph nodes metastasis: analysis of 2,269 Chinese breast cancer patients treated with extended radical mastectomy. Breast Cancer Res Treat. 2008; 107:379–387.
Article27. Lacour J, Bucalossi P, Cacers E, Jacobelli G, Koszarowski T, Le M, et al. Radical mastectomy versus radical mastectomy plus internal mammary dissection: five-year results of an international cooperative study. Cancer. 1976; 37:206–214.
Article28. Livingston SF, Arlen M. The extended extrapleural radical mastectomy: its role in the treatment of carcinoma of the breast. Ann Surg. 1974; 179:260–265.29. Coombs NJ, Boyages J, French JR, Ung OA. Internal mammary sentinel nodes: ignore, irradiate or operate? Eur J Cancer. 2009; 45:789–794.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recurrent Encapsulated Papillary Carcinoma in the Ipsilateral Internal Mammary Lymph Node: a Case Report
- Contralateral Internal Mammary Lymphadenopathy Mimicking Metastasis in a Patient with a History of Breast Cancer and Prior Interstitial Mammoplasty by Paraffin Injection: MRI, PET-CT, and Pathological Findings
- Diagnosis of a Malignant Intramammary Node Retrospectively Aided by Mastectomy Specimen MRI-Is the Search Worth It? A Case Report and Review of Current Literature
- Magnetic Resonance Findings of Breast Diseases
- A Systematic Review on Radiologists' Knowledge of Breast Cancer Screening